Abstract
Background
Topical medication is the mainstay for treatment of mild psoriasis. However, dissatisfaction with topicals is common and rates of non-adherence are high. Assessing patients’ perspectives can help to identify unmet needs.
Objective
Our aim was to investigate satisfaction of patients with psoriasis with topical therapy and to determine influencing factors.
Methods
Patients were recruited from the Department of Dermatology, University Medical Center Mannheim, Germany. Satisfaction was assessed using the Treatment Satisfaction Questionnaire for Medication version 1.4 with the domains effectiveness, side effects, convenience, and global satisfaction (scale 0–100 each). The impact of sociodemographic and disease characteristics was determined by multivariate regression.
Results
Averaged across the cohort (n = 122, mean age 52.5 years, 58.2% male), the side effects domain had the highest mean satisfaction score (89.7), followed by convenience (72.5), global satisfaction (60.8), and effectiveness (55.0). Comparing specific medications, combinations of corticosteroids and vitamin D analogues were rated best in effectiveness. Treatment satisfaction was influenced by age, partnership, ability to apply topicals independently, disease-related quality-of-life impairment, sole or adjunctive use of topicals and pruritus.
Conclusions
Participants were particularly satisfied with safety but rather dissatisfied with effectiveness of topicals. Topical therapy should be adapted to individual needs with special attention to effectiveness.
Keywords:
Introduction
Psoriasis, a chronic inflammatory disease, affects about 2–4% of the population in Western industrial countries (Citation1,Citation2). Although a large number of highly effective systemic therapies are available for patients with moderate-to-severe psoriasis, topical therapy remains a central component in the management of the disease (Citation2). Approximately, 78–90% of patients with psoriasis suffer from mild to moderate disease, which can often be adequately controlled with topical therapy alone (Citation3–5). Furthermore, topicals can be used as adjunctive therapy for patients on phototherapy or systemic treatment (Citation4). The range of currently available topical treatment options includes topical corticosteroids (TCS), vitamin D analogues (VDA), fixed combinations of TCS and VDA, anthralin (dithranol), topical calcineurin inhibitors (TCI), salicylic acid, and moisturizers (Citation6). Despite these numerous options, overall satisfaction with topical therapy is limited (Citation7,Citation8). Both primary adherence (redemption of the prescription) and secondary adherence (using the treatment correctly) are poor (Citation9). The reasons given in the literature include frustration over low effectiveness, suboptimal cosmetic acceptability, high complexity of treatment regimes, and side effects (Citation10–12).
Investigating patient satisfaction with topical treatment is of great importance to increase adherence and consequently improve therapeutic success as well as overall quality of life (Citation8,Citation9,Citation13,Citation14). The aim of our study was to investigate the satisfaction of patients with psoriasis with topical therapy in general and with specific topical medications, and to determine patient and disease-related influencing factors.
Materials and methods
Study cohort
Patients with psoriasis of all levels of severity visiting the in- and outpatient Department of Dermatology of the University Medical Center Mannheim, Germany were recruited between March 2015 and September 2017. Inclusion criteria were a physician-confirmed diagnosis of plaque psoriasis, current treatment with topical therapy (either solely or in combination with other modalities), age ≥18 years, ability to provide informed consent and German language skills. This non-interventional study was approved by the Ethics Committee of the Medical Faculty Mannheim and was performed according to the principles of the Declaration of Helsinki (ethics approval 2009-329E-MA, 22 October 2009; amendment 5 February 2015).
Data collection
After providing written informed consent, the participants completed a survey with questions on age, sex, partnership status, current main topical therapy, other topical therapies, other treatment modalities (phototherapy, traditional systemic therapy, and/or biologicals), ability to apply the topical therapy independently and pruritus. Options for topical therapy comprised TCS, VDA, combinations of TCS and VDA, salicylic acid, urea, anthralin, TCI, and vitamin A agonists. Moreover, respondents could indicate another medication as free text, or chose ‘unknown’ if they did not remember the specific drug. For all medications, both generic and brand names were listed, and multiple responses were possible. Treatment satisfaction with the topical therapy currently applied predominantly was assessed using the Treatment Satisfaction Questionnaire for Medication (TSQM) version 1.4 (German translation) (Citation15), a validated and reliable instrument that consists of 14 items and results in four specific domains: effectiveness, side effects, convenience, and global satisfaction (Citation15). Scores for each domain range from 0 to 100, with higher scores representing higher satisfaction for the respective domain. Furthermore, participants completed the Dermatology Life Quality Index (DLQI) (Citation16). The Psoriasis Area and Severity Index (PASI) (Citation17) of each participant was scored by S.H. or M.-L.S.
Statistical analyses
Treatment satisfaction of the entire cohort was calculated using the TSQM version 1.4. Associations between TSQM scores and participants’ characteristics, in particular, sex, age, partnership status (with partner vs. without partner), PASI, DLQI, ability to apply topical therapy independently (yes vs. no), use of topical medications as sole or adjunctive therapy, and pruritus (yes vs. no) were assessed with descriptive statistics. Differences in means between groups were tested for statistical significance with the Mann–Whitney U-test or an unpaired t-test. Associations between continuous variables and satisfaction scores were examined using Pearson’s correlations (PC). In addition, multivariate linear regression analysis was performed to investigate the independent influence of characteristics on satisfaction. The models contained the TSQM domains as dependent variables and sex, age, PASI, DLQI, partnership status, the ability to apply topical therapy independently, sole use of topical treatment and pruritus as independent variables.
Furthermore, minimum, maximum, median, mean, and 25th percentile (lower quartile) as well as 75th percentile (upper quartile) of TSQM domains were calculated for specific topical medications. The Shapiro–Wilk test revealed normal distribution for the effectiveness domain, but not for the other domains. Therefore, differences in scores of the effectiveness domain between the specific agents were explored using analysis of variance (ANOVA), while the Mann–Whitney U-test with Bonferroni correction was used for the other domains.
All statistical analyses were carried out with the software SAS (Statistical Analysis System, SAS Institute Inc., Cary, NC), Version 9.4. The graphs were created with GraphPad Prism Version 9.4.1 (La Jolla, CA). Statistical significance was assumed for p values <.05 for all tests.
Results
Study population
One hundred and twenty-three patients agreed to participate in the study. One patient had to be excluded due to a largely incomplete questionnaire. Thus, 122 patients were included in the final analyses. The mean age of the cohort was 52.5 years, and 58.2% of the participants were male. The mean DLQI was 11.6, indicating an overall significant impact of psoriasis on quality of live, whereas the mean PASI was relatively low (5.8), likely because most participants received treatment at the time of data collection. 59.8% were living in a partnership. Most participants were able to apply topical therapy independently (86.1%), and 60.7% reported to suffer from pruritus.
At the time of study participation, topical treatment was applied as the sole therapy in 28.8% and as adjunctive treatment in 71.2% of the participants. 34.8% received phototherapy, 24.6% traditional systemic therapy, and 33.1% biologicals ().
Table 1. Characteristics of the study cohort.
Patients’ satisfaction with topical antipsoriatic therapy
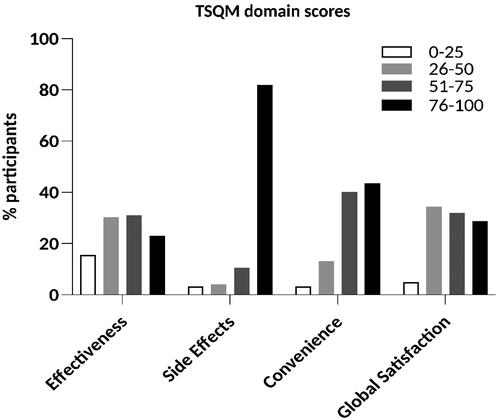
Averaged across all study participants, the side effects domain was rated with the highest score (mean (standard deviation (SD)): 89.7 (20.7)), followed by convenience (mean (SD): 72.5 (23.9)), global satisfaction (mean (SD): 60.8 (22.5)), and effectiveness (mean (SD): 55.0 (26.4); range: 0–100 for all domains). The distribution of the scores in each domain is shown in . 82.0% of the scores of the side effects domain, but only 43.4% of the convenience domain, 28.7% of the global satisfaction domain, and 23.0% of the effectiveness domain were above 75.
Associations between participants’ characteristics and treatment satisfaction
Higher age was associated with higher convenience scores in descriptive analysis (PC: 0.24; p = .007; ), but this result was not confirmed in our regression analysis (). Stratification according to sex revealed no significant differences in any of the four TSQM domains (, ). Interestingly, however, patients living with a partner reported higher TSQM scores in the side effects domain (93.8 vs. 83.7, p = .007, ; regression model: β = 11.069; p = .010, ) and in the convenience domain (78.7 vs. 63.2, p < .001, ; regression model: β = 13.078; p = .003, ) than those without a partner. Current objective disease severity, measured by the PASI, did not impact satisfaction in any of the four TSQM domains (, ). However, the DLQI correlated inversely with the TSQM domains convenience (PC: −0.37; p < .001; ; regression model: β = –0.770; p = .016, ) and global satisfaction (PC: −0.20; p = .022; ; regression model: β = –0.666; p = .043, ), indicating that a higher disease-related quality-of-life impairment was associated with lower satisfaction in these domains. Participants who were able to apply topical therapy independently scored significantly higher in the convenience domain than those who were not able to do so (75.4 vs. 54.8, p = .002; ; regression model: β = 17.444; p = .003, ). Patients with pruritus were less satisfied with the convenience of their therapy than those without pruritus according to the descriptive analysis (67.6 vs. 79.9, p = .005, ), but this observation could not be verified in the regression model (). Participants who used topical agents as adjunctive therapy reported higher TSQM scores in the global satisfaction domain than those using topicals as sole therapy (50.7 vs. 65.7, p = .002, ; regression model: β = 16.955; p < .001, ).
Figure 2. Associations between TSQM scores and age, sex and partnership. (a) Higher age was associated with higher convenience scores (PC: 0.24; p = .007). (b) No significant differences were found with respect to sex. (c) Participants living with a partner scored higher in the side effects (93.8 vs. 83.7, p = .007) and convenience (78.7 vs. 63.2, p < .001) domains. Bars: PC (a) or means with standard deviations (b, c). PC: Pearson’s correlation coefficient. *p < .05, **p < .01, and ***p < .001.
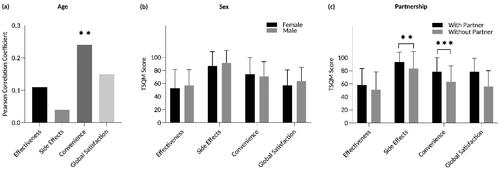
Figure 3. Associations between TSQM scores and PASI and DLQI. (a) There were no significant correlations between PASI and any of the four TSQM domains. (b) DLQI scores and the TSQM domains convenience (PC: –0.37; p < .001) and global satisfaction (PC: –0.20; p = .022) correlated inversely. Bars: PC. DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index; PC: Pearson’s correlation coefficient. *p < .05 and ***p < .001.
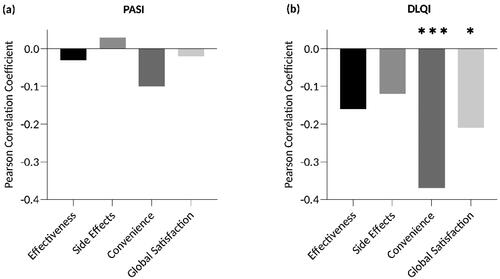
Figure 4. Associations between TSQM scores and the ability to apply topical therapy independently, pruritus and sole or adjunctive use of topical treatment. Satisfaction with convenience was significantly higher when patients were able to apply topical therapy independently (75.5 vs. 54.8, p = .002; (a)) and when pruritus was absent (67.6 vs. 79.9, p = .005; (b)). (c) Global satisfaction was higher in patients who used topicals as adjunctive therapy (50.7 vs. 65.7, p = .002). Bars: means with standard deviations. **p < .01.
Table 2. Multiple linear regression models showing associations of participants’ characteristics with treatment satisfaction with the currently prescribed topical treatment.
Satisfaction with specific topical antipsoriatic medications
Satisfaction with the topical therapy currently applied predominantly was analyzed; however, 61.5% of patients used at least one further topical therapy (). Most often anthralin (22.1%), VDA monotherapy (20.5%), TCS monotherapy (19.7%), and fixed combinations of TCS and VDA (14.8%) were stated as predominant therapy in the presented study (). Subgroup analysis on specific medications revealed a significantly higher mean satisfaction score in the effectiveness domain for fixed combinations of TCS and VDA compared to TCS monotherapy (mean: 69.1 vs. 51.9, median: 67.0 vs. 50.0, p = .027), VDA monotherapy (mean: 69.1 vs. 53.8, median: 67.0 vs. 56.0, p = .047), urea (mean: 69.1 vs. 44.8, median: 67.0 vs. 47.0, p = .005), and salicylic acid (mean: 69.1 vs. 33.3, median: 67.0 vs. 28.0, p < .001; ). Furthermore, patients treated with VDA monotherapy (mean: 53.8 vs. 33.3, median: 56.0 vs. 28.0, p = .044) or anthralin (mean: 61.9 vs. 33.3, median: 67.0 vs. 28.0, p = .005) had significantly higher satisfaction scores in the effectiveness domain than patients treated with salicylic acid (). Patients receiving anthralin reported higher satisfaction with effectiveness than those applying urea (mean: 61.9 vs. 44.8, median: 67.0 vs. 47.0, p = .030; ).
Figure 5. Satisfaction with specific topical medications. (a) Higher scores in the effectiveness domain were found for the combination therapy of TCS and VDA compared to TCS monotherapy (mean: 69.1 vs. 51.9, median: 67.0 vs. 50.0, p = .027), VDA monotherapy (mean: 69.1 vs. 53.8, median: 67.0 vs. 56.0, p = .047), urea (mean: 69.1 vs. 44.8, median: 67.0 vs. 47.0, p = .005), and salicylic acid (mean: 69.1 vs. 33.3, median: 67.0 vs. 28.0, p < .001). Treatment with VDA monotherapy (mean: 53.8 vs. 33.3, median: 56.0 vs. 28.0, p = .044) or anthralin (mean: 61.9 vs. 33.3, median: 67.0 vs. 28.0, p = .005) was associated with higher satisfaction scores in the effectiveness domain compared to salicylic acid therapy. Furthermore, patients treated with anthralin were more satisfied with the effectiveness than those treated with urea (mean: 61.9 vs. 44.8, median: 67.0 vs. 47.0, p = .030). (b) The combination of TCS and VDA was associated with a higher mean score in the side effects domain than salicylic acid (mean: 96.9 vs. 66.4, median: 100.0 vs. 66.0, p < .001). (c) Patients with VDA monotherapy scored significantly higher in the convenience domain compared to those with anthralin therapy (mean: 82.0 vs. 59.7, median: 83.0 vs. 67.0, p < .001). (d) No statistically significant differences between agents were detected in the global satisfaction domain. The box bounds the interquartile range (upper quartile–lower quartile) divided by the median (horizontal line) and whiskers extend to the minimum and maximum of the data values. The cross (+) indicates the mean value. TCS: topical corticosteroids; VDA: vitamin D analogues; TCS + VDA: fixed combination of TCS and VDA. *p < .05, **p < .01, and ***p < .001.
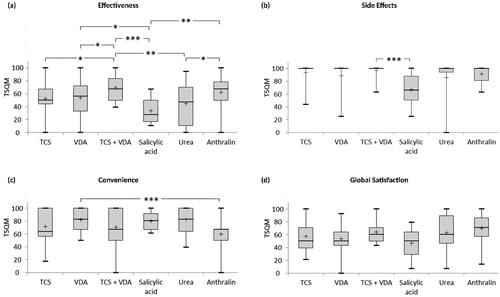
Table 3. Kind of current topical therapy.
Satisfaction with side effects was significantly higher for combinations of TCS and VDA compared to salicylic acid (mean: 96.9 vs. 66.4, median: 100.0 vs. 66.0, p < .001; ). Satisfaction with convenience was higher for VDA monotherapy than for anthralin (mean: 82.0 vs. 59.7, median: 83.0 vs. 67.0, p < .001; ). Global satisfaction scores did not differ significantly between the specific topical antipsoriatic medications ().
Discussion
We performed a monocentric non-interventional study in which we assessed patients’ satisfaction with topical antipsoriatic therapy in general and with the whole range of topical medications currently available in Germany in a real-world setting. Averaged across all participants, satisfaction with the effectiveness of topical therapies was rated worst of all TSQM domains. Less than a quarter of the participants achieved a score between 75 and 100, corresponding to high satisfaction. This finding is in line with the literature, according to which patients often perceive their topical therapy as not effective enough (Citation8). However, satisfaction with the effectiveness of topical therapy varies greatly between individuals and also depends significantly on the therapeutic agent used (Citation7,Citation11,Citation18–20). In our previous study on patient preferences for topical psoriasis treatments, participants considered efficacy the most important aspect of their therapy (Citation21). The strong desire for an effective therapy on the one hand and the low level of satisfaction reached for the effectiveness domain on the other hand emphasize an unmet need.
Satisfaction with the side effects of topical agents was very high in our cohort. In line with this finding, another study of our group revealed higher satisfaction with side effects of topical therapy compared to phototherapy, traditional systemic therapy and biologicals in a cohort of patients with moderate-to-severe psoriasis (Citation7). Compared to other treatment modalities, side effects of topical therapy are often transient, mild and mostly limited to the skin (e.g., skin irritation, pruritus, skin atrophy, telangiectasia, and striae), while systemic side effects as well as severe adverse events are extremely rare (Citation22–25).
The convenience domain reached the second highest average score, even if less than half of our participants were very satisfied in this domain. The relatively high level of satisfaction with convenience is somewhat surprising, as patients often describe their topical therapy as inconvenient, complex, and time-consuming (Citation10,Citation12,Citation26). When interpreting this finding, it has to be kept in mind that a large proportion of our cohort used topical medications as adjunctive therapy and that the average PASI score was low. Subgroup analysis revealed that participants treated exclusively with topical therapy were less satisfied with its convenience than patients using topical agents adjunctively. Clearly, it is more convenient to apply topical therapy only on a few, refractory psoriasis lesions than to use it as exclusive treatment on large parts of the body.
According to descriptive analysis, younger age was associated with lower convenience scores. It can be assumed that the time-consuming and/or inconvenient application of topical antipsoriatics has a more profound impact on social and professional activities of younger patients. Concordantly, a study by Gupta and Gupta (Citation27) demonstrated that problems related to appearance, socialization, occupation, and finances were more common in psoriasis patients younger than 45 years compared to older ones. Maul et al. (Citation28) analyzed needs and expectations of patients with psoriasis from the German and Swiss registries PsoBest and Swiss Dermatology Network of Targeted Therapies and detected higher issues regarding social impairments in younger patients. These observations underscore that it is particularly important to adapt the topical therapy of younger patients to individual needs and to evaluate the satisfaction with the convenience at follow-up visits.
Patients living with a partner and participants who were able to apply the topical therapy independently reported higher convenience than others. In a study conducted by Iversen and Jakobsen (Citation29), 80% of the patients with psoriasis had at least one affected body part where topical treatment was difficult to apply. Therefore, it is comprehensible that the presence of a partner who can help with application as well as the ability to use the therapy without assistance favorably influence satisfaction with convenience. Prior to prescription of topical medications and during follow-up consultations, it should be discussed with the patient whether an independent application is possible or whether support is available.
Participants living with a partner also had higher TSQM scores in the side effects domain than participants living alone. It is well conceivable that the handling of side effects is facilitated by the support of a partner. In line with this assumption, it was demonstrated in patients with cancer that the negative effects of pain were decreased in a partnership (Citation30).
DLQI scores correlated inversely with the TSQM domains convenience and global satisfaction, which is well plausible. Health-related quality of life of patients with a chronic disease and satisfaction with the therapy are mutually influencing factors. On the one hand, a severely impaired quality of life may trigger depressive moods and dissatisfaction in general. On the other hand, dissatisfaction with the therapy has a negative impact on quality of life (Citation31).
Participants without pruritus scored significantly higher in the convenience domain. Topical treatments, in particular TCS, may contribute to reducing itch, a symptom commonly reported by patients with psoriasis. Therefore, patients who experienced relief from their itch may be particularly satisfied with the convenience of their therapy. On the other hand, pruritus is a common side effect of several topical therapies (VDA, urea, salicylic acid, and anthralin), which may lead to further dissatisfaction in patients who already suffer from itch (Citation32).
Satisfaction with specific antipsoriatic medications
Comparing specific topical antipsoriatic treatments, fixed combinations of TCS and VDA were rated best in the effectiveness and side effects domains. This is not surprising considering that, on the one hand, the combination has synergistic effects in terms of effectiveness and, on the other hand, it leads to a reciprocal reduction regarding the side effects of TCS and VDA monotherapy (Citation6,Citation33–35). A recent study assessed efficacy and safety of proactive psoriasis management with twice-weekly calcipotriene/betamethasone dipropionate foam over 52 weeks (Citation36). The combination was well tolerated, and no skin atrophy was observed (Citation36). It is therefore not only suitable as an effective initial therapy, but also constitutes an appropriate option for proactive long-term use (Citation6,Citation37).
Topical salicylic acid achieved the lowest scores in the side effects domain. Due to its keratolytic properties, salicylic acid reduces scaling and softens psoriatic plaques (Citation4). It is therefore particularly beneficial in the initial phase of psoriasis therapy or in case of refractory thick and scaly plaques. However, it can irritate the skin, and it may not be used on a large body surface because of systemic absorption (Citation4).
Anthralin was rated worst in the convenience domain. This is not surprising, because anthralin therapy is complex and sometimes uncomfortable. The concentration has to be increased over time, depending on the patient’s response and tolerance. The therapy frequently leads to erythema and burning as well as to discoloration of the skin, clothes, and patient environment. Therefore, anthralin therapy is often performed in an inpatient or day clinic setting, where the medication is applied by experienced medical staff (Citation6). However, anthralin shows high response rates, and rates of treatment failure are low (Citation38). This might explain the relatively high global satisfaction with anthralin, as effectiveness is known to be one of the most important impact factors on treatment satisfaction (Citation18–20).
Taken together, satisfaction scores for specific medications show clear differences. Patients’ individual satisfaction with regard to effectiveness, side effects, and convenience should be evaluated during clinical visits in order to optimize the therapy if necessary.
Novel and emerging topical agents for psoriasis
With further understanding of psoriasis pathogenesis, novel targeted topical therapies are emerging, which will expand the treatment armamentarium in the future (Citation39). For example, aryl hydrocarbon receptor (AhR) modulators, inhibitors of the phosphodiesterase type 4 (PDE-4) and the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathways as well as retinoic acid receptor‑related orphan receptor‑γ (RORγ) agonists seem to be promising topical strategies for chronic plaque psoriasis (Citation39). In May 2022, tapinarof, an AhR modulator, was approved by the FDA for topical treatment of plaque psoriasis in adults (Citation40). Tapinarof 1% cream, applied once daily, demonstrated good efficacy in two phase III studies including more than 1000 patients, with 35.4% (PSORING 1) or 40.2% (PSORING 2) of the participants achieving a Physician’s Global Assessment (PGA) score of 0 or 1 and a decrease from baseline of at least two points at week 12 (Citation39). Two phase III randomized vehicle-controlled studies evaluated the efficacy and safety of topical roflumilast, a PDE-4 inhibitor, once daily and found an Investigator Global Assessment (IGA) success (clear or almost clear status plus 2-grade improvement from baseline) of 42.4% (DERMIS-1) or 37.5% (DERMIS-2) after 8 weeks (Citation41). It will be interesting to investigate treatment satisfaction with these novel agents compared to the more established topical antipsoriatic medications in the future.
Limitations
Our study was performed in a monocentric setting in a German university hospital, and the cohort size was limited. Therapies were self-reported by the participants. Participants were asked to state treatment satisfaction with the currently predominantly used topical therapy. However, several participants (50.8%) used more than one topical agent. In addition, more than 70% of the patients received topical medications in combination with other modalities (phototherapy, traditional systemic therapies, and/or biologicals). Therefore, it cannot be excluded that the satisfaction scores reported for the predominant topical therapy were influenced by the satisfaction with the whole treatment regime. The severity of psoriasis was assessed with the PASI, which is the standard method for monitoring the severity of psoriasis in clinical trials (Citation17). However, the score has its limitations, especially for recording the severity of psoriasis in patients with mild-to-moderate disease activity (Citation42). Although we assessed the impact of several variables on satisfaction, further confounders might exist which could not be evaluated in this study. For example, the affection of specific body areas (scalp, facial, intertriginous, genital, palmar, or plantar regions), the skin type and ethnicity were not recorded, but may also influence satisfaction. Last but not least, the formulation of agents (solution, cream, ointment, foam, or gel) has considerable impact on patient preferences for and satisfaction with topical drugs (Citation43). According to a systematic review, most patients with psoriasis preferred less messy and less oily topical drug formulations such as foams, solutions, and lotions (Citation43).
Conclusions
We show that patients with psoriasis are particularly satisfied with the safety, but rather dissatisfied with the effectiveness of topical therapy. Satisfaction with specific medications differed significantly in several domains, and fixed combinations of TCS and VDA were rated best in the domain effectiveness. Moreover, we identified several patient and disease characteristics that influence satisfaction. Our results demonstrate the importance of individualized topical treatment regimens as well as the need for novel effective topical agents. Satisfaction with topical treatments should be evaluated regularly, and reasons for dissatisfaction should be exactly elucidated. Adaptation of topical therapy to individual needs with special attention to effectiveness may increase satisfaction, which probably leads to improved adherence and, thereby, to higher treatment efficacy.
Acknowledgements
The authors would like to thank all participants.
Disclosure statement
N. Ninosu, S. Hoelker, M. Kappenstein, and S. Buettner declare no conflicts of interest. W. K. Peitsch served as advisor for and/or obtained speakers’ honoraria from and/or received grants from and/or participated in clinical trials by the following companies: AbbVie, ALK-Abello, Almirall Hermal, Array Biopharma, Beiersdorf, Biotest, BMS, Boehringer Ingelheim, Celgene, Dermapharm, Dermasence, Eli Lilly, Galderma, GSK, Janssen-Cilag, L’Oreal, La Roche Posay, LEO Pharma, Medac, MSD, Novartis, Pfizer, Dr. Pfleger, Pierre Fabre, P&M Cosmetics, Roche, Sanofi, Sun Pharma, and UCB Pharma. M.-L. Schaarschmidt has been an advisor to and/or received speakers’ honoraria from and/or received grants from and/or participated in clinical trials by the following companies: Abbvie, Allmirall, Biogen Inc., BMS GmbH, Böhringer-Ingelheim, Celgene, Eli Lilly, Janssen-Cilag GmbH, Merck Serono GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH, and UCB. The disclosed conflicts of interest have not influenced the content of this manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Wu JJ, Feldman SR, Koo J, et al. Epidemiology of mental health comorbidity in psoriasis. J Dermatolog Treat. 2018;29(5):1–9.
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470.
- Papp KA, Gniadecki R, Beecker J, et al. Psoriasis prevalence and severity by expert elicitation. Dermatol Ther. 2021;11(3):1053–1064.
- Körber A, Wilsmann-Theis D, Augustin M, et al. Topische Therapie bei Psoriasis vulgaris – ein Behandlungspfad [Topical therapy for psoriasis vulgaris. A treatment pathway]. J Dtsch Dermatol Ges. 2019;17(S4):3–14.
- Schaarschmidt M-L, Kromer C, Herr R, et al. Treatment satisfaction of patients with psoriasis. Acta Derm Venereol. 2015;95(5):572–578.
- Florek AG, Wang CJ, Armstrong AW. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res. 2018;310(4):271–319.
- Devaux S, Castela A, Archier E, et al. Adherence to topical treatment in psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(3):61–67.
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139.
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55(4):607–613.
- Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl. 4):9–14.
- Thorneloe RJ, Bundy C, Griffiths CEM, et al. Adherence to medication in patients with psoriasis: a systematic literature review. Br J Dermatol. 2013;168(1):20–31.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease – strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33(12):2253–2263.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a National Panel Study of Chronic Disease. Health Qual Life Outcomes. 2004;2:12.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24(2):10–16.
- Krueger G, Koo J, Lebwohl M, et al. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001;137(3):280–284.
- Dubertret L, Mrowietz U, Ranki A, et al. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155(4):729–736.
- van Cranenburgh OD, Korte JD, Sprangers MAG, et al. Satisfaction with treatment among patients with psoriasis: a web-based survey study. Br J Dermatol. 2013;169(2):398–405.
- Hoelker S, Ninosu N, Buettner S, et al. Patient preferences for topical psoriasis treatments: a discrete choice experiment. J Dermatolog Treat. 2022;33(5):2595–2604.
- Bruner CR, Feldman SR, Ventrapragada M, et al. A systematic review of adverse effects associated with topical treatments for psoriasis. Dermatol Online J. 2003;9(1):2.
- Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54(4):383–392.
- Bakshi H, Nagpal M, Singh M, et al. Treatment of psoriasis: a comprehensive review of entire therapies. Curr Drug Saf. 2020;15(2):82–104.
- Nast A, Altenburg A, Augustin M, et al. German S3-guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm – part 2: treatment monitoring and specific clinical or comorbid situations. J Dtsch Dermatol Ges. 2021;19(7):1092–1115.
- Feldman SR. Approaching psoriasis differently: patient–physician relationships, patient education and choosing the right topical vehicle. J Drugs Dermatol. 2010;9(8):908–911.
- Gupta MA, Gupta AK. Age and gender differences in the impact of psoriasis on quality of life. Int J Dermatol. 1995;34(10):700–703.
- Maul J-T, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33(4):700–708.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6(2):273–285.
- Morgan MA, Small BJ, Donovan KA, et al. Cancer patients with pain: the spouse/partner relationship and quality of life. Cancer Nurs. 2011;34(1):13–23.
- Hjortsberg C, Bergman A, Bjarnason A, et al. Are treatment satisfaction, quality of life, and self-assessed disease severity relevant parameters for patient registries? Experiences from Finnish and Swedish patients with psoriasis. Acta Derm Venereol. 2011;91(4):409–414.
- Nast A, Boehncke WH, Mrowietz U, et al. German S3-guidelines on the treatment of psoriasis vulgaris (short version). Arch Dermatol Res. 2012;304(2):87–113.
- Kragballe K, Gjertsen BT, De Hoop D, et al. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991;337(8735):193–196.
- Menter A, Gold LS, Bukhalo M, et al. Calcipotriene plus betamethasone dipropionate topical suspension for the treatment of mild to moderate psoriasis vulgaris on the body: a randomized, double-blind, vehicle-controlled trial. J Drugs Dermatol. 2013;12(1):92–98.
- Yan R, Jiang S, Wu Y, et al. Topical calcipotriol/betamethasone dipropionate for psoriasis vulgaris: a systematic review. Indian J Dermatol Venereol Leprol. 2016;82(2):135–144.
- Lebwohl M, Kircik L, Lacour J-P, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J Am Acad Dermatol. 2021;84(5):1269–1277.
- Hoegsberg T, Iversen L, Lange MM, et al. Topical treatment of psoriasis: questionnaire results on topical therapy as long-term continuous treatment and use on specific body sites. J Dermatolog Treat. 2021;32(8):916–921.
- Painsi C, Patscheider M, Inzinger M, et al. Patient perspectives on treating psoriasis with classic inpatient dithranol therapy: a retrospective patient survey. J Dtsch Dermatol Ges. 2015;13(11):1156–1163.
- Lé AM, Torres T. New topical therapies for psoriasis. Am J Clin Dermatol. 2022;23(1):13–24.
- Keam SJ. Tapinarof cream 1%: first approval. Drugs. 2022;82(11):1221–1228.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328(11):1073–1084.
- Svendsen MT, Andersen KE. The significance of the Lattice-System Physician’s Global Assessment as a research tool for measuring mild-to-moderate psoriasis. J Am Acad Dermatol. 2022;86(3):e111–e112.
- Svendsen MT, Feldman SR, Tiedemann SN, et al. Psoriasis patient preferences for topical drugs: a systematic review. J Dermatolog Treat. 2021;32(5):478–483.