Abstract
Background
Differences in atopic dermatitis (AD) disease course and manifestation with age may extend to treatment response.
Objective
To evaluate response maintenance with continuous-/reduced-dose abrocitinib or withdrawal and response to treatment reintroduction after flare in adolescent and adult participants in JADE REGIMEN (NCT03627767).
Methods
Adolescents (12–17 years) and adults with moderate-to-severe AD responding to abrocitinib 200-mg induction were randomly assigned to 40-week maintenance with abrocitinib (200 mg/100 mg) or placebo. Patients who experienced flare during maintenance received rescue treatment.
Results
Of 246 adolescents and 981 adults, 145/246 (58.9%) and 655/981 (66.8%), respectively, responded to induction. Similar proportions of adolescents and adults experienced flare during maintenance with abrocitinib 200 mg (14.9%/16.9%), 100 mg (42.9%/38.9%), and placebo (75.5%/78.0%). From the abrocitinib 200-mg, 100-mg, and placebo arms, respectively, Eczema Area and Severity Index response was recaptured by 28.6%, 25.0%, and 52.9% of adolescents and 34.3%, 33.7%, and 58.0% of adults; Investigator’s Global Assessment response, by 42.9%, 50.0%, and 73.5% of adolescents and 34.3%, 50.6%, and 74.1% of adults. Abrocitinib had a similar safety profile regardless of age; nausea incidence was higher in adolescents.
Limitations
Adolescents represented 20% of the trial population.
Conclusion
Abrocitinib was effective in preventing flare in adolescents and adults.Clinicaltrials.gov listing: NCT03627767.
Introduction
Atopic dermatitis (AD) is a relapsing-remitting, inflammatory skin disease characterized by eczematous lesions, intense itch, and impaired quality of life (QOL) (Citation1–5). AD develops in approximately 12% of children aged 6 months to <12 years and in 15% aged 12 to <18 years (Citation1,Citation6). Childhood-onset AD commonly resolves before adulthood; however, approximately 5% of patients diagnosed before the age of 18 continue to experience AD for at least another 20 years (Citation7). The 12-month prevalence of AD in US adults is 5% (Citation8), and 53% of US adults with AD report onset during adulthood (Citation9), indicating a complex and variable disease course.
The predilection sites of lesions, trigger factors, skin microbiome, and underlying pathophysiology of AD differ in pediatric and adult patients (Citation10,Citation11). In infants, lesions are widely distributed on the face and the trunk; in adolescents and adults, flexural involvement is more common (Citation1). The microbiome of nonlesional skin is significantly more diverse in pediatric than in adolescent or adult patients with AD (Citation11), and the cytokine expression profile in the skin of individuals with AD fluctuates with the age of the patient (Citation12). Physiology also varies in an age-dependent manner; altered skin structure and function lead to a vulnerable epidermal barrier and a higher risk of absorption of topical drugs in young children than in adults (Citation10). This age-related diversity of AD pathophysiology suggests that treatment response may also vary with the age of the patient.
JADE REGIMEN was conducted to evaluate response to abrocitinib, an oral, once-daily, Janus kinase 1 (JAK1)-selective inhibitor, over a period of 40 weeks, with continuous treatment (200 mg), dose reduction (100 mg), or withdrawal in patients who initially had a short-term response to treatment with abrocitinib 200 mg (Citation13). In this post hoc analysis, we evaluated results for adolescent and adult participants in JADE REGIMEN to identify potential differences by age.
Methods
Patients
Eligible patients were aged ≥12 years (body weight ≥40 kg) and had moderate-to-severe AD (Investigator’s Global Assessment [IGA] score ≥3, Eczema Area and Severity Index [EASI] score ≥16, percentage of body surface area (%BSA) affected ≥10, and Peak Pruritus Numerical Rating Scale [PP-NRS; used with permission from Regeneron Pharmaceuticals, Inc., and Sanofi] score ≥4) for ≥1 year. Patients had a recent history (≤6 months) of inadequate response to topical medicated therapy or had previously required systemic therapy. Full details of the inclusion and exclusion criteria were published previously (Citation13).
Study design
JADE REGIMEN was conducted in agreement with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice Guidelines. This research was approved by institutional review boards or ethics committees at each site. Internal and external review committees monitored the safety of patients throughout the study. All patients provided written informed consent.
JADE REGIMEN was a multicenter, responder-enriched, double-blind, placebo-controlled, randomized withdrawal, phase 3 trial that comprised three periods:
An open-label induction period (12 weeks) to determine response to abrocitinib 200 mg once daily. Response to induction was defined as achieving an IGA score of 0 (clear) or 1 (almost clear) with ≥2-grade improvement from baseline (IGA 0/1 response) and ≥75% improvement from baseline in EASI (EASI-75 response)
A double-blind, randomized, maintenance-withdrawal period (40 weeks) in which responders were assigned (1:1:1) to receive abrocitinib 200 mg, abrocitinib 100 mg, or placebo as monotherapy. Randomization was stratified by age (<18 and ≥18 years)
An open-label rescue period (12 weeks) in which patients who had experienced the loss of response (i.e., flare, defined as ≥50% loss of the initial week 12 EASI response and a new IGA score ≥2) during the maintenance period received abrocitinib 200 mg plus topical medicated therapy (e.g., topical corticosteroids, calcineurin inhibitors, or crisaborole)
Endpoints
The primary endpoint of JADE REGIMEN was the proportion of patients who had a loss of response (i.e., flare) during the maintenance period and required rescue treatment (7). This post hoc analysis assessed the proportion of patients achieving EASI-75, IGA 0/1, and ≥4-point improvement from study baseline in PP-NRS (PP-NRS4) during the open-label induction period. The proportion of patients who showed ≥4-point improvement from the study baseline in Children’s Dermatology Life Quality Index (CDLQI4; adolescent patients aged 12–17 years) or Dermatology Life Quality Index (DLQI4; adult patients aged ≥18 years) was evaluated during the randomized maintenance period. Higher CDLQI/DLQI scores represent a larger impact of AD on QOL; the lowest scores (0 or 1) represent no effect, and the highest range (CDLQI, 19–30; DLQI, 21–30) represents an extremely large effect (Citation14,Citation15). Also assessed were the proportions of patients who experienced the loss of response (i.e., flare) during the maintenance period and subsequently recaptured EASI and IGA response with rescue treatment (abrocitinib 200 mg + topical medicated therapy) during the rescue period. Recapture of response was defined as achieving an EASI/IGA score during the rescue period not worse than EASI/IGA score at randomization baseline (if available), otherwise achieving an EASI/IGA score not worse than EASI/IGA score at week 12. Safety was assessed via adverse event (AE) monitoring, and laboratory values were recorded for both adolescents and adults in a standardized manner.
Statistical analysis
For this post hoc analysis, data were analyzed for adolescents (12–17 years) and adults (≥18 years). Confidence intervals (CIs) for the probability of loss of response were derived using the log-log transformation with back transformation to an untransformed scale. CIs for Kaplan–Meier estimate of time to flare were based on the Brookmeyer and Crowley method (Citation16). Hazard ratios (HRs) and CIs were estimated from Cox proportional hazards regression models, including fixed effects of treatment, categorical variables of randomization strata (age category), and disease severity at study baseline in the main model, and a continuous variable of weight at study baseline added as a covariate in the sensitivity analysis. The study baseline was defined as the last measurement collected on or before day 1 of trial treatment.
Results
Patient demographics and baseline disease characteristics
A total of 246 adolescents and 987 adults were treated in the open-label induction period (Supplemental Figure S1). The mean age (SD) was 15.1 years (1.8 years) in the adolescent group and 35.7 years (13.8 years) in the adult group (). Similar proportions of adolescents and adults were Asian (15.4%, 16.0%), Black or African American (9.3%, 5.3%), and White (72.8%, 76.2%). Disease duration was shorter in adolescents (median [Q1–Q3], 12.8 years [8.2–15.0]) than in adults (21.5 years [10.3–31.5]). Similar proportions of adolescents and adults had moderate disease at baseline based on IGA score (56.5%, 59.8%). EASI scores at baseline were comparable in adolescents (30.0 [21.6–40.9]) and adults (27.5 [20.8–37.0]), as were %BSA values (50.0% [33.0–67.8], 44.8% [30.5–62.0]). A greater proportion of adolescents than adults received treatment with only topical agents before the trial (46.3%, 37.8%); systemic treatment with biologic agents was more common in adult patients; 8.0% of adults had exposure before the trial compared with 2.8% of adolescent patients.
Table 1. Patient demographics and baseline characteristics.
Efficacy
Open-label induction period
The rate of response (IGA 0/1 with ≥2-grade improvement from baseline and EASI-75) was numerically lower in adolescents (58.9% [95% CI, 52.8–65.1]) than adults (66.8% [63.8–69.7]) after 12 weeks of treatment with abrocitinib 200 mg; however, CIs overlapped (Supplemental Figure S2). PP-NRS4 response rates were lower in adolescents (57.5%; 95% CI, 50.0–65.0) than adults (70.6%; 67.4–73.8). IGA 0/1 response was achieved by 59.8% (95% CI, 53.6–65.9) of adolescents and 67.5% (64.6–70.4) of adults and EASI-75 response by 71.5% (65.9–77.2) of adolescents and 76.6% (73.9–79.2) of adults (Supplemental Figure S2).
Randomized maintenance period
After the response to induction, 47, 49, and 49 adolescents and 219, 216, and 218 adults were randomly assigned to the abrocitinib 200-mg, abrocitinib 100-mg, and placebo arms, respectively. Disease flare (≥50% loss of week 12 response per EASI and new IGA score ≥2) occurred in similar proportions of adolescents and adults who received abrocitinib 200 mg (14.9% and 16.9%), abrocitinib 100 mg (42.9% and 38.9%), or placebo (75.5% and 78.0%).
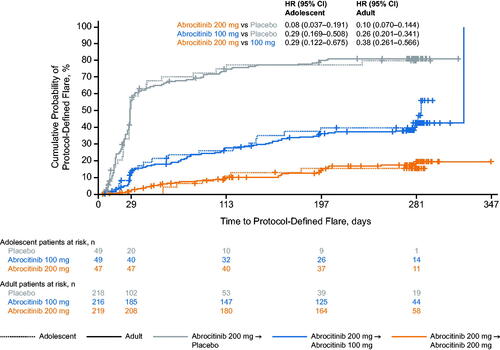
The cumulative probability of disease flare by week 52 in each treatment arm was similar between adolescents and adults: 15.3% (95% CI, 7.6–29.5), 42.7% (29.6–58.6), and 79.7% (66.9 − 90.0) for adolescents and 19.6% (14.4–26.4), 42.8% (35.7–50.7), and 81.2% (75.5 − 86.2) for adults, respectively, who received abrocitinib 200 mg, abrocitinib 100 mg, and placebo (). Kaplan-Meier estimates of median time to flare were 285.0 d (95% CI, 140.0 to not evaluable) in adolescents and 323.0 d (281.0–323.0) in adults in the abrocitinib 100-mg arm and 28.0 d (27.0–42.0) in adolescents and 28.0 d (28.0–30.0) in adults in the placebo arm. Median time to flare could not be estimated for abrocitinib 200 mg for either age group because of low event rates. The risk of flare with abrocitinib 200 mg versus 100 mg was numerically lower in adolescents (HR, 0.29 [95% CI, 0.12–0.68]) than in adults (0.38 [0.26–0.57]); however, CIs overlapped. The risk of the flare was comparable between adolescents and adults for abrocitinib 200 mg versus placebo (adolescents, 0.08 [95% CI: 0.04–0.19]; adults, 0.10 [0.07–0.14]) and abrocitinib 100 mg versus placebo (0.29 [0.17–0.51]; 0.26 [0.20–0.34]). The HR for flare in adolescents versus adults in all treatment groups was approximately 1 (placebo, 1.01 [95% CI, 0.71–1.44]; abrocitinib 100 mg, 1.16 [0.72–1.87]; abrocitinib 200 mg, 0.85 [0.38–1.90]), indicating a similar risk between adolescents and adults in each treatment arm. In both adolescents and adults, the risk of flare among treatment groups was not altered by adjusting for body weight (data not shown).
Figure 1. Probability of protocol-defined flare for adolescent and adult patients during the randomized maintenance period of the JADE REGIMEN study. Flare was defined in the protocol as ≥50% loss of initial EASI response at week 12 with a new IGA score ≥2. Patients at risk were defined as patients who did not experience flare and were continuing treatment. Missing event times were considered as right censored (censored at random) on the last date of randomized treatment. Patients included in the adolescent group were aged 12–17 years. Patients included in the adult group were aged ≥18 years. CI: confidence interval; EASI: Eczema Area and Severity Index; HR: hazard ratio; IGA: Investigator’s Global Assessment.
Rescue period
A larger proportion of adolescents (49.0%) than adults (39.4%) from the abrocitinib 100-mg maintenance arm entered the rescue period. Similar proportions of adolescents and adults entered the rescue period from the abrocitinib 200-mg (14.9% and 16.9%) and placebo (77.6% and 78.0%) maintenance arms.
After 12 weeks of rescue treatment (abrocitinib 200 mg with medicated topical therapy, including corticosteroids, calcineurin inhibitors, and crisaborole), the EASI response recapture rate was numerically lower in adolescents than in adults who entered the rescue period from all treatment arms of the maintenance period; however, CIs overlapped. EASI response was recaptured by 28.6% (95% CI, 0.0–62.0), 25.0% (6.0–44.0), and 52.9% (36.2–69.7) of adolescents from the abrocitinib 200-mg, abrocitinib 100-mg, and placebo maintenance arms, respectively, compared with 34.3% (18.6–50.0), 33.7% (23.6–43.9), and 58.0% (50.4–65.6) of adults (Supplemental Figure S3). Conversely, the IGA response recapture rate was numerically higher in adolescents than adults who entered the rescue period from the abrocitinib 200-mg treatment arm, with overlapping CIs (42.9% [95% CI, 6.2–79.5] and 34.3% [18.6–50.0]), and similar in patients who entered the rescue period from the abrocitinib 100-mg maintenance arm (50.0% [28.1–71.9] and 50.6% [39.8–61.4]) and the placebo maintenance arm (73.5% [58.7–88.4] and 74.1% [67.3–80.8]).
Quality of life
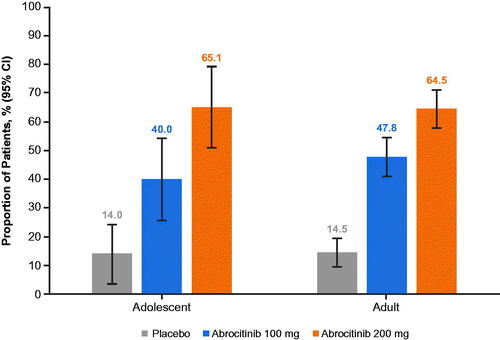
At the study baseline, median CDLQI/DLQI scores were 12.0 (moderate effect) in adolescents and 16.0 in adults (very large effect). At the randomization baseline, approximately 95% of adolescents and adults achieved ≥4-point improvement in response per the CDLQI/DLQI from the study baseline (CDLQI4/DLQI4; data not shown). By week 52, the respective proportions of adolescent and adult patients who achieved improvement per the CDLQI4/DLQI4 were 65.1% and 64.5% in the abrocitinib 200 mg arm, 40.0% and 47.8% in the abrocitinib 100 mg arm, and 14.0% and 14.5% in the placebo arm ().
Figure 2. Proportion of patients who achieved ≥4-point improvement from study baseline in health-related quality-of-life index score at week 40 of the randomized maintenance period of the JADE REGIMEN study. Quality of life of adolescents (12–17 years old) was assessed using the CDLQI. Quality of life of adult patients (≥18 years old) was assessed using the DLQI. Study baseline was defined as the last measurement collected on or before day 1 of treatment. Patients who withdrew from the trial or experienced flare and received rescue treatment after randomization were counted as not having achieved the outcome after withdrawal. CDLQI: Children’s Dermatology Life Quality Index; CI: confidence interval; DLQI: Dermatology Life Quality Index.
Safety
Similar proportions of adults and adolescents reported treatment-emergent adverse events (TEAEs) during the open-label induction period (Supplemental Table S1); the severity of most TEAEs was mild to moderate. Serious adverse events (SAEs) occurred in 1.2% of adolescents and 1.7% of adults during the open-label induction period.
In the maintenance period, the frequencies of TEAEs were 68.1%, 59.2%, and 44.9% in adolescents randomly assigned to receive abrocitinib 200 mg, abrocitinib 100 mg, and placebo, respectively, compared with 62.1%, 52.8%, and 45.4% in adults (). SAEs occurred in 4.3% and 2.0% of adolescents who received abrocitinib 200 mg and placebo. No SAEs were observed in adolescents who received abrocitinib 100 mg. In adults, the frequencies of SAEs were 5.0%, 1.9%, and 0.5% with abrocitinib 200 mg, 100 mg, and placebo, respectively. Rates of discontinuation due to TEAEs were 4.3%, 0%, and 2.0% in adolescents and 6.4%, 2.3%, and 1.4% in adults who received abrocitinib 200 mg, abrocitinib 100 mg, and placebo, respectively.
Table 2. Summary of patient-year and incidence rates for TEAEs (any causality) for adolescents and adults treated in the randomized maintenance period of JADE REGIMEN.
The incidence rates (95% CI) for nausea were 39.49 (21.59–66.26), 21.04 (9.08–41.46), and 30.55 (13.97–58.00) events per 100 patient-years (PY) in adolescents in the abrocitinib 200-mg, abrocitinib 100-mg, and placebo arms, respectively, compared with 19.33 (13.46–26.88), 15.26 (10.06–22.20), and 26.33 (18.34–36.62) events per 100 PY in adults. For acne or folliculitis, incidence rates (95% CI) were 14.23 (5.22–30.97), 20.99 (9.06–41.35), and 22.36 (8.99–46.08) events per 100 PY in adolescents who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo, respectively, compared with 16.98 (11.61–23.97), 14.23 (9.29–20.85), and 13.79 (8.42–21.29) events per 100 PY in adults.
Platelet counts decreased from baseline during the induction period, reaching a nadir at week 4 in both adolescent and adult patients. During the maintenance phase, platelet counts returned to near baseline levels in adolescents who received abrocitinib 100 mg but not in adults who received abrocitinib 100 mg; platelet counts remained low in adolescents and adults who received continued-dose abrocitinib 200 mg (Supplemental Figure S4). Despite fluctuations, median platelet counts remained within the clinically normal range (150,000–450,000) throughout the trial. Platelet count abnormalities led to treatment discontinuation in one adult patient during the induction period and in zero patients during the maintenance period. No clinically significant changes were observed in the other laboratory values measured in either adolescents or adults (data not shown).
Discussion
Abrocitinib was effective and well tolerated by adolescents and adults throughout the JADE REGIMEN study. More than half the adolescents (59%) and two-thirds of adults (67%) achieved an IGA 0/1 and EASI-75 response with abrocitinib 200 mg by week 12 of the induction period and were randomly assigned to the maintenance period. During the randomized maintenance period, abrocitinib prevented flare more effectively than placebo in both adolescents and adults. The risk of the flare was similar for adolescents and adults in all treatment arms. IGA 0/1, EASI-75, and PP-NRS4 response rates in adolescents and adults in the induction period were consistent with the overall population (Citation13).
Our findings show that abrocitinib efficacy was comparable between adolescents and adults. This may be due to its mechanism of action; selective blocking of JAK1 inhibits the signaling of a broad panel of pro-inflammatory cytokines, including interleukin (IL)-4, IL-13, and thymic stromal lymphopoietin (Citation17). Upregulation of these cytokines has been shown to occur to a similar extent in the lesional skin of adolescent and adult patients with AD compared with the skin of age-matched controls (Citation12).
Treatment with abrocitinib also resulted in improvements in patient QOL. At week 40 of the maintenance period, the majority of both adolescent (65%) and adult (64%) patients receiving abrocitinib 200 mg reported a ≥ 4-point improvement from baseline in CDLQI/DLQI. These results are consistent with other reports of approved therapies for AD in similar patient populations; clinically meaningful improvements were achieved in DLQI scores (defined as minimal clinically important difference [MCID] of at least 4 points from baseline) with upadacitinib, baricitinib, and dupilumab, and CDLQI scores (MCID of at least 6–8 points from baseline) with dupilumab (Citation18–23). Health-related QOL data from randomized controlled trials are lacking for adolescent patients with AD and represent a significant unmet need (Citation24). The findings from this study support the hypothesis that the use of effective therapies can improve QOL outcomes in adolescents with AD, a patient population in whom significant levels of anxiety, depression, and poor sleep quality along with disease symptoms contribute to a profound negative impact on their daily lives (Citation3,Citation25–27).
The safety profile of abrocitinib was generally consistent in adolescents and adults, with some differences. The overall frequency of TEAEs was higher in adolescents than adults during the induction period. The incidence rates of nausea during the randomized maintenance period were also numerically higher in adolescents than adults, albeit with overlapping CIs. Despite these differences, rates of discontinuation due to TEAEs were low during the induction and maintenance periods and comparable in both age groups.
The key strength of this analysis is the evaluation of efficacy, safety, and QOL outcomes across different abrocitinib dosing regimens from a phase 3 study in a well-defined population of both adults and adolescents with AD. This study also had limitations. Differences observed between age groups in this analysis should be interpreted with caution due to the small sample size of the adolescent group (only 20% of the total JADE REGIMEN trial population). Slight imbalances were noted in the previous treatment characteristics between adolescents and adults: adolescent patients were more likely to have received only topical agents before day 1 of the trial, whereas adult patients were treated using a broader armamentarium, including systemic immunomodulatory agents. The differences in efficacy, safety, and QOL outcomes between adolescent and adult patients were assessed post hoc and not powered for statistical significance. The extent of the statistical comparison was limited and risk factors for flare could not be determined.
Conclusions
This post hoc analysis of the JADE REGIMEN study confirms the efficacy and safety of abrocitinib in adolescents and adults over a period of 40 weeks of treatment, although disease response rates for some outcomes seemed numerically lower in adolescents. These results reinforce that treatment with abrocitinib using either induction with abrocitinib 200 mg followed by maintenance with reduced-dose abrocitinib 100 mg, or continuous dosing with abrocitinib 200 mg is an effective therapeutic approach in adults and adolescents with moderate-to-severe AD.
Supplemental Material
Download PDF (476.7 KB)Acknowledgments
Editorial/medical writing support under the guidance of the authors was provided by Megan K. Elder, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022;10.7326/M22-1460).
Disclosure statement
C Flohr is an investigator with funding from the European Union Horizon 2020 Program, the UK-Irish Atopic Eczema Systemic Therapy Register (A-STAR), and UK National Institute for Health and Care Research (NIHR), has provided unpaid advice to Pfizer Inc., AbbVie, Eli Lilly and Company, LEO Pharma, and Sanofi-Genzyme, and holds investigator-led funding from Pfizer Inc. and Sanofi-Genzyme. MJC is an investigator for Pfizer Inc., Atopix, Galapagos Pharmaceutical Company, Hyphens Pharma, Johnson & Johnson, Kymab, LEO Pharma, L’Oréal/La Roche-Posay, Novartis, Regeneron, and Sanofi-Genzyme and an advisory board member/consultant/speaker for Pfizer Inc., AbbVie, Amlar, Astellas, Atopix, Boots, Dermavant, Galapagos Pharmaceutical Company, Galderma, Hyphens Pharma, Johnson & Johnson, Kymab, LEO Pharma, L’Oréal/La Roche-Posay, Menlo Therapeutics, Novartis, Oxagen, Procter & Gamble, Reckitt Benckiser, Regeneron, and Sanofi-Genzyme. MRAJ is a speaker/consultant/advisor/research collaborator for Pfizer Inc., AbbVie, Amgen, Ducentis BioTherapeutics, Sosei Heptares, LEO Pharma, Sanofi-Genzyme, and Unilever. LFE is an adviser/consultant/clinical study investigator for Pfizer Inc., AbbVie, Almirall, Amgen, Arcutis, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly, and Company, Forté, Galderma, Glenmark/Ichnos, Incyte, LEO Pharma, Novartis, Ortho Dermatologics, Regeneron, and Sanofi-Genzyme. SB has served as a scientific adviser/consultant/clinical study investigator for Pfizer Inc., AbbVie, Almirall, Eli Lilly and Company, Janssen, Laboratoire La Roche Posay, LEO Pharma, Pierre Fabre Laboratory, Novartis, Sanofi-Genzyme, and UCB Pharma. C Feeney and JN are employees and stockholders of Pfizer Inc. RR is a former employee and a current stockholder of Pfizer Inc. IL is an employee of IQVIA Pharmaceuticals and was a paid contractor to Pfizer Inc. for manuscript development and statistical support.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10.e2–7.
- Ezzedine K, Shourick J, Merhand S, et al. Impact of atopic dermatitis in adolescents and their parents: a French study. Acta Derm Venereol. 2020;100(17):adv00294.
- Misery L, Finlay AY, Martin N, et al. Atopic dermatitis: impact on the quality of life of patients and their partners. Dermatology. 2007;215(2):123–129.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59(4):e75–e91.
- Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol. 1999;103(1 pt 1):125–138.
- Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(4):681–687.e11.
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293.
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J Am Acad Dermatol. 2019;80(6):1526.e7–1532.e7.
- Fölster-Holst R. Management of atopic dermatitis: are there differences between children and adults? J Eur Acad Dermatol Venereol. 2014;28(Suppl 3):5–8.
- Shi B, Bangayan NJ, Curd E, et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol. 2016;138(4):1233–1236.
- Renert-Yuval Y, Thyssen JP, Bissonnette R, et al. Biomarkers in atopic dermatitis-a review on behalf of the international eczema council. J Allergy Clin Immunol. 2021;147(4):1174.e1–1190.e1.
- Blauvelt A, Silverberg JI, Lynde CW, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 atopic dermatitis efficacy and safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. 2022;86(1):104–112.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664.
- Waters A, Sandhu D, Beattie P, et al. Severity stratification of children’s dermatology life quality index (CDLQI) scores. Br J Dermatol. 2010;163(Suppl 1):121.
- Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29–41.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293–304.
- Basra MKA, Salek MS, Camilleri L, et al. Determining the minimal clinically important difference and responsiveness of the dermatology life quality index (DLQI): further data. Dermatology. 2015;230(1):27–33.
- Simpson EL, de Bruin-Weller M, Eckert L, et al. Responder threshold for patient-oriented eczema measure (POEM) and children’s dermatology life quality index (CDLQI) in adolescents with atopic dermatitis. Dermatol Ther. 2019;9(4):799–805.
- Chiricozzi A, Gori N, Narcisi A, et al. Effectiveness and safety of upadacitinib in the treatment of moderate-to-severe atopic dermatitis: a multicentric, prospective, real-world, cohort study. Drugs R D. 2022;22(3):245–252.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35(7):1543–1552.
- Paller AS, Bansal A, Simpson EL, et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: post-hoc analyses from a randomized clinical trial. Am J Clin Dermatol. 2020;21(1):119–131.
- Silverberg JI, Simpson EL, Boguniewicz M, et al. Dupilumab provides rapid and sustained clinically meaningful responses in adults with moderate-to-severe atopic dermatitis. Acta Derm Venereol. 2021;101(11):adv00585.
- Cork MJ, McMichael A, Teng J, et al. Impact of oral abrocitinib on signs, symptoms and quality of life among adolescents with moderate-to-severe atopic dermatitis: an analysis of patient-reported outcome. J Eur Acad Dermatol Venereol. 2022;36(3):422–433.
- Ricci G, Bellini F, Dondi A, et al. Atopic dermatitis in adolescence. Dermatol Reports. 2012;4(1):e1.
- Teixeira C, Garcia MJ, Freitas A, et al. Impact of atopic dermatitis on the mental health of adolescents: literature review. Med Sci Forum. 2022;16(8):1–5.
- Grant L, Larsen LS, Trennery C, et al. Conceptual model to illustrate the symptom experience and humanistic burden associated with atopic dermatitis in adults and adolescents. Dermatitis. 2019;30(4):247–254.

