Dear Editor,
Atopic dermatitis (AD) is a chronic inflammatory skin disease principally characterized by type 2 inflammation, skin barrier dysfunction, and itch (Citation1). In developed countries, it affects almost 30% of children, being the most frequently reported chronic inflammatory skin disorder of infancy (Citation1). However, it is estimated that the prevalence of AD among adolescents (aged 13–17 years) ranges from 0.2% to 24.6% globally (Citation2). AD is commonly associated with other atopic conditions such as asthma, food allergies and allergic rhinitis (Citation1).
The pathogenesis of AD is still not entirely understood and depends on many factors, leading to different treatment approaches. A key role is played by the activation of Th2 lymphocytes with the release of interleukin (IL)-4, IL-5, IL-13, and IL-31 (Citation1).
The gold standard therapy for mild-to-moderate AD includes emollients, topical glucocorticoids, and topical calcineurin inhibitors. In children and adolescents with moderate-to-severe AD, according to European Guidelines, the available systemic treatment options are cyclosporine, dupilumab and upadacitinib (in license for ≥ 16, 6 and 12 years, respectively) (Citation3,Citation4). In particular, dupilumab is a human monoclonal antibody which targets alpha subunit of the interleukin (IL)-4-Receptor (ILRα), inhibiting both the IL-13 and IL-4 pathways (Citation5).
We report our experience on 30 patients aged between 12 and 18 years old who received dupilumab to assess its effectiveness and safety profiles. Effectiveness was assessed in terms of reduction of 75% and 90% in the Eczema Area and Severity Index (EASI) (EASI 75 and EASI 90, respectively) after 16 and 52 weeks. We also evaluated the reduction of the Pruritus-Numerical Rating Scale (P-NRS) and Sleep loss-NRS (S-NRS) throughout the study period.
According to the summary of product characteristics, eight patients initially received a dosage of 400 mg at day one, followed by 200 mg every two weeks, while the other 22 patients received a dosage of 600 mg at day one followed by 300 mg every two weeks, given their weight greater than 60 kg (Citation6).
All patients completed at least 16 weeks of treatment, and 25 reached one year. The mean age was 16.1 years with a Standard Deviation (SD) of 2.07, and 66.67% of all patients were females. The most common atopic comorbidities were allergic asthma and allergic rhinitis (10 patients each). Regarding sensitive areas, the most frequently involved was the face (23 patients), followed by the hands (6 patients).
Concerning the previous systemic treatment, the most prescribed drugs were cyclosporine (8 patients), followed by systemic corticosteroids (7) and upadacitinib (2). The clinical features and demographic characteristics of the patients are shown in
Table 1. Clinical features and demographic characteristics of the patients.
The mean EASI decreased from 23.25 (SD 4.15) at baseline to 4.5 (4.52) at week 16 and 2.06 (1.99) at week 52 (). At week 16, EASI 75 and EASI 90 were reached 66.67% and 36.67%, respectively. After one year, out of 25 patients, 96,67% and 73,33% achieved EASI75 and EASI90 ().
Figure 1. mEASI (top left), EASI75 and EASI90 (top right), S.NRS (bottom left) and P-NRS (bottom right) throughout the study period. mEASI: mean eczema area and severity index; S-NRS: sleep numerical rating scale; P-NRS: pruritus numerical rating scale
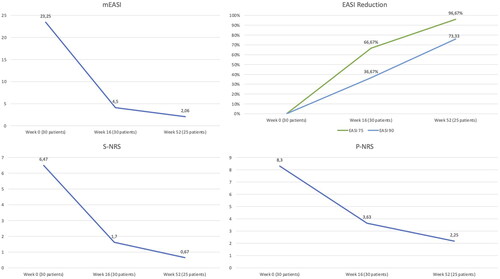
The mean P-NRS score was 8.3 (SD 1.44) at baseline, and it descended to 3.63 (2.78) and 2.25 (2.19) at weeks 16 and 52, respectively (). Regarding insomnia, we observed a decrease in mean S-NRS from 6.47 (2.85) to 1.7 (2.45) at week 16 and 0.67 (1.40) at week 52 ().
Regarding the safety profile, one patient developed bilateral conjunctivitis and two patients experienced facial redness. No severe adverse events (AEs) or AEs leading to discontinuation were observed in our study.
Dupilumab is one of the few systemic options approved for the treatment of moderate-to-severe AD in adolescents and real-life experiences are currently limited. We observed excellent clinical responses, as EASI 75 and EASI 90 were higher than that observed in both clinical trials (Citation5,Citation7) and comparable to real-life experiences (Citation8–10). At week 52, we observed even better outcomes in terms of EASI75 and EASI90 than a real-life experience from Stingeni et al. (Citation11).
In our experience, the safety profile of dupilumab was excellent, with a lower rate of adverse events compared with both clinical trials and real-life experiences.
In conclusion, our experience, although limited, showed that dupilumab is a safe and effective therapeutic option for a real-life cohort of adolescents. Larger observational studies, are needed to confirm the effectiveness and safety of dupilumab in adolescents affected by AD.
Compliance with ethics guidelines
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received dupilumab as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Acknowledgements
None.
Disclosure statement
L. Gargiulo has received research grants from Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly and Boehringer Ingelhei. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):1–3.
- Ezzedine K, Shourick J, Merhand S, et al. Impact of atopic dermatitis in adolescents and their parents: a French study. Acta Derm Venereol. 2020;100(17):adv00294. Published 2020 Oct 20.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431.
- Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of Upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther . 2023;13(2):651–660.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56.
- European Medicines Agency. Dupixent (dupilumab): summary of product characteristics. 2023. [cited 2023 February 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_it.pdf.
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23(3):365–383.
- Pagan AD, David E, Ungar B, et al. Dupilumab improves clinical scores in children and adolescents with moderate to severe atopic dermatitis: a real-world, single-center study. J Allergy Clin Immunol Pract. 2022;10(9):2378–2385.
- Stingeni L, Bianchi L, Antonelli E, et al. Moderate-to-severe atopic dermatitis in adolescents treated with dupilumab: a multicentre Italian real-world experience. J Eur Acad Dermatol Venereol. 2022;36(8):1292–1299.
- Gargiulo L, Piscazzi F, Ibba L, et al. Dupilumab for the treatment of atopic dermatitis of the elderly: a real-life 52-week experience. J Dermatolog Treat. 2023;34(1):2192840. DOI:10.1080/09546634.2023.2192840
- Stingeni L, Bianchi L, Antonelli E, et al. A 52-week update of a multicentre Italian real-world experience on effectiveness and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37(3):e384–e388.

