Abstract
Aim
To compare real-world dose escalation of risankizumab with other US Food and Drug Administration (FDA)-approved biologic treatments for management of moderate-to-severe psoriasis (PsO) in the United States.
Methods
The Merative® MarketScan® Research Database was used to identify adults with ≥2 medical claims for PsO, ≥3 claims of the index biologic medication in the maintenance period, and ≥6 months continuous enrollment pre-induction and ≥6 months after initiation of the maintenance period. Dose escalation was defined as ≥2 dosing intervals where the average daily dose was ≥30% higher than the expected daily dose (per FDA-approved dosing). Comparisons between risankizumab and other cohorts were made using chi-square tests and logistic regression models.
Results
At the 30% threshold, the percentage of patients with dose escalation in the full maintenance period was significantly lower with risankizumab (2.0%) compared with other drug classes (tumor necrosis factor, interleukin (IL)-12/23, IL-17, or other IL-23 inhibitors: 17.6%, 10.0%, 18.3%, or 7.1%, respectively; p < 0.0001 for each) and individual biologics (adalimumab, ustekinumab, secukinumab, ixekizumab, and guselkumab; 17.9%, 10.0%, 15.7%, 18.0%, and 7.2%, respectively; p < 0.0001).
Conclusion
A significantly lower proportion of risankizumab-treated patients with PsO had dose escalations compared with patients treated with other biologics.
Keywords:
Introduction
Psoriasis (PsO) is a chronic inflammatory disease with a range of symptoms affecting the skin. In the United States, the prevalence of PsO is estimated at 3%, corresponding to approximately 6.9 million adults in 2019, with approximately half of cases being moderate to severe (Citation1–4). Moderate-to-severe PsO is often treated with biologic inhibitors targeting interleukin (IL)-23, IL-12/23, IL-17, and tumor necrosis factor (TNF) (Citation1). Despite the availability of advanced biologic treatments, a subset of patients do not reach target goals of high skin clearance or lose response to treatment over time (Citation5,Citation6).
In clinical practice, dose escalation may be considered to improve the effectiveness of biologics in patients with an inadequate response (Citation7–9) and is common practice globally (Citation9–12). Dose escalation involves increasing the dose received and/or decreasing the interval between doses. In clinical trial data, escalating dosing temporarily in patients with PsO who have had an inadequate response to adalimumab demonstrated an improvement in symptoms of PsO at Week 24, which were maintained to Week 108 (Citation13). At Week 12, 25% of patients with PsO had reached a 50% reduction in Psoriasis Area and Severity Index (PASI) scores after dose escalation of adalimumab, and by Week 24, 35% of patients achieved a 50% reduction in PASI (Citation14).
Risankizumab, a humanized immunoglobulin G1 monoclonal antibody that binds to the p19 subunit of IL-23, was approved for use by the US Food and Drug Administration (FDA) in patients with moderate-to-severe PsO in 2019 (Citation15,Citation16). In Phase 3 trials, risankizumab has demonstrated superior efficacy in skin clearance and patient-reported outcomes at Week 16 compared with placebo and ustekinumab in patients with moderate-to-severe plaque PsO (Citation16). Additionally, significantly greater percentages of patients receiving risankizumab versus placebo had a treatment response at Week 16 which was maintained up to 2 years, demonstrating the durability of the response to risankizumab (Citation17). A recent open-label extension interim analysis reported that continuous risankizumab use over 172 weeks was well tolerated and showed high and durable efficacy, further supporting the benefit of risankizumab (Citation18).
In previous real-world studies, dose escalation was estimated in up to two-thirds of patients receiving biologics including adalimumab, etanercept, infliximab, secukinumab, or ustekinumab (Citation9,Citation11); however, these studies did not evaluate therapies approved over the last several years. This study aimed to compare real-world dose escalation during risankizumab treatment versus other FDA-approved biologics for the management of moderate-to-severe PsO in the United States.
Methods
Study design and participants
This was a real-world, retrospective cohort analysis that examined data from the Merative® MarketScan® Research Databases (2018–2021) with Early View data until 31 July 2021. These data include health insurance claims across the continuum of care (e.g., inpatient, outpatient, pharmacy, as well as enrollment data), a variety of fee-for-service, preferred provider organizations, and capitated and Medicare supplemental health plans. The study design is illustrated in .
Adult patients (≥18 years) who initiated risankizumab, TNF inhibitors (adalimumab, etanercept, or certolizumab), other IL-23 inhibitors (guselkumab or tildrakizumab), IL-12/23 inhibitors (ustekinumab), or IL-17 inhibitors (secukinumab, ixekizumab, or brodalumab) between 23 April 2019, and 30 June 2021, were eligible to be included. Patients had to have a confirmed PsO diagnosis (≥2 PsO diagnoses on distinct dates on or before the induction period index date [the date of the first claim for index biologic], with at least one PsO diagnosis in the 6 months on or before the induction index date) based on International Classification of Diseases (ICD)-9 (696.1) and ICD-10 (L40.0, L40.1, L40.2, L40.3, L40.4, L40.8, L40.9) codes. Patients were also required to have ≥3 claims of an index biologic in the maintenance period (the period after completion of the induction treatment plus 7 days; ). Continuous eligibility of medical and pharmacy insurance was required ≥6 months prior to the induction index date and ≥6 months after initiation of the maintenance period.
Table 1. Baseline demographics and characteristics at MOA Level.
Patients with other immune-mediated conditions (rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, Crohn’s disease, hidradenitis suppurativa, uveitis, or psoriatic arthritis diagnosis at baseline) and patients with index biologic use in the baseline period before the first prescription claim in the induction period were excluded.
Baseline characteristics
Patient characteristics (age, sex, region, insurance type), comorbidities (Charlson Comorbidity Index [CCI]), and previous treatment history were measured in the 6 months before (and including) the index date for the induction period.
Outcomes
The study evaluated dose escalation rates in the maintenance period of biologic treatment. Dose escalation was defined as ≥2 dosing intervals where the average daily dose (total strength [milligrams] divided by days between subsequent doses) was ≥30% higher than the expected daily dose (EDD) during the maintenance period (Citation9,Citation19). The EDD during the maintenance period was based on product-specific dosing as per the FDA label () (Citation20–29). If patients had ≥2 prescription intervals above the dose escalation threshold, they were identified as prevalent cases of dose escalation. Drug quantity used in the dosing evaluation was based on quantities billed to the patient’s health plan. Quantities were increased for risankizumab (two instead of one) when the strength in the claims was 75 mg for the original formulation. Dose escalation was evaluated for the entire maintenance period (until the end of treatment duration and/or continuous eligibility) as well as the first 6 and 12 months of the maintenance period.
Prescription claims for patients were excluded from the analysis after any evidence of non-persistence (defined as a gap in therapy that exceeded 1.5 times the dosing interval/prescription fill for a standard maintenance dose). Prescription fills occurring within 4 days of another prescription fill or prescription fills with extreme values (i.e., >3 times the EDD) were presumed errors in the data and were also excluded.
Statistical analyses
Comparisons in dose escalation were made between risankizumab and other drug classes (TNF inhibitors, IL-12/23 inhibitors, IL-17 inhibitors, other IL-23 inhibitors) and other individual biologics (with a sample size of >50 patients). P values were reported based on chi-square tests.
Adjusted odds ratios and 95% confidence intervals (CIs) were derived from logistic regression models adjusting for age, gender, region, CCI, previous biologic or targeted immunomodulator (TIM) use at baseline, and weight status. Sensitivity analysis for dose escalation was also conducted using a 20% threshold for escalation.
Ethical requirements
Data provided for the study were de-identified and Health Insurance Portability and Accountability Act (HIPAA) compliant; therefore, review board approval was not required.
Results
Study population
A total of 3383 patients were included in the full maintenance period analysis, out of which 2919 and 1336 patients had ≥6 months and ≥12 months treatment duration, respectively (). The mean age of patients was 44.9 ± 12.4 in the risankizumab cohort versus 44.6 ± 13.3, 45.5 ± 12.9, 46.1 ± 11.9, and 46.3 ± 11.8 years in the IL-12/23 inhibitors, TNF, other IL-23, and IL-17 inhibitors cohorts, respectively (). Of patients in the risankizumab cohort, 157/744 (21.1%) had previously used biologics, compared with 39/740 (5.3%) in the TNF cohort, 47/349 (13.5%) in IL-12/23 cohort, 194/815 (23.8%) in the IL-17 cohort, and 183/735 (24.9%) in the other IL-23 inhibitors cohort. Baseline characteristics by individual biologics were consistent to the class level ().
Dose escalation: risankizumab versus biologics with other mechanisms of action (MOAs)
At the MOA level, the percentage of patients with dose escalation in the full maintenance period was significantly lower with risankizumab (2.0%) than with other IL-23, IL-12/23, TNF, or IL-17 inhibitors (7.1%, 10.0%, 17.6%, or 18.3%, respectively) at the 30% threshold (all p < 0.0001; ). Trends in results were similar for the first 6 and 12 months of the maintenance period (all p < 0.01, respectively). Patients in the risankizumab cohort had significantly lower adjusted odds ratios of dose escalation at the 30% threshold () than patients treated with other IL-23, IL-12/23, TNF, and IL-17 inhibitors (all p < 0.0001).
Figure 2. Proportion of patients with ≥2 escalated dosing intervals above the 30% threshold during the maintenance period by (A) MOA and (B) Biologic. **p < 0.01, ***p < 0.0001 compared to risankizumab based on chi-square test. IL: interleukin; MOA: mechanism of action; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
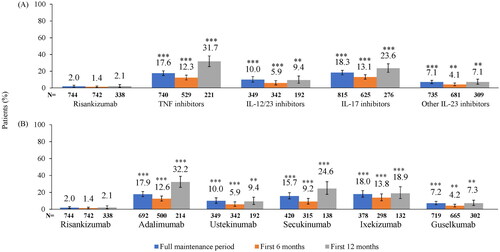
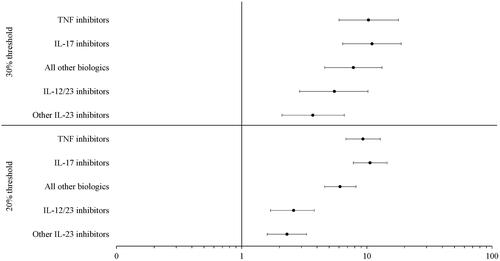
Figure 3. Adjusted odds ratios of dose escalation at the 30% and 20% thresholds by MOA. All p < 0.0001. CI: confidence interval; IL: interleukin; MOA: mechanism of action; OR: odds ratio; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
Dose escalation: risankizumab versus other biologics
At the individual biologic level, the mean duration of maintenance treatment for risankizumab was 366.1 days compared with secukinumab, adalimumab, ixekizumab, guselkumab, and ustekinumab which were 304.4, 305.7, 315.0, 352.1, and 406.0 days, respectively. The percentage of patients with dose escalation was significantly lower with risankizumab (2.0%) than with guselkumab, ustekinumab, secukinumab, adalimumab, and ixekizumab (7.2%, 10.0%, 15.7%, 17.9%, and 18.0%, respectively) at the 30% threshold (p < 0.0001; ). Trends in results were similar for the first 6 and 12 months of the maintenance period (p < 0.0001 and p < 0.01, respectively). Patients in the risankizumab cohort had significantly lower adjusted odds ratios of dose escalation at the 30% threshold () than patients treated with guselkumab, ustekinumab, secukinumab, adalimumab, and ixekizumab (all p < 0.0001).
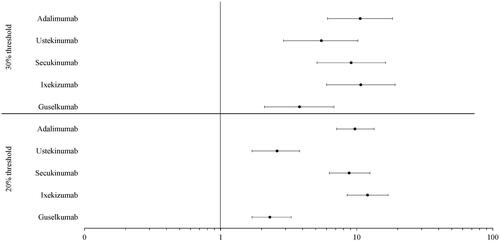
Figure 4. Adjusted odds ratios of dose escalation at the 30% and 20% thresholds by biologic. All p < 0.0001. CI: confidence interval; IL: interleukin; OR: odds ratio.
The average number of dose escalated claims in the full maintenance period for risankizumab was 2.3 compared with 2.7, 3.0, 3.3, 3.4, and 4.1 for secukinumab, guselkumab, ustekinumab, ixekizumab, and adalimumab, respectively ().
Sensitivity analysis: dose escalation with a 20% threshold
At the MOA of biologics level, the percentage of patients with dose escalation was significantly lower with risankizumab (7.1%) than with other IL-23, IL-12/23, TNF, and IL-17 inhibitors (15.1%, 16.1%, 41.2%, and 44.7%, respectively) at the 20% threshold (all p < 0.0001; ). Results were similar for the first 6 and 12 months of the maintenance period (all p < 0.0001 for the first 6 months and all p < 0.05 for the first 12 months). Patients in the risankizumab cohort had significantly lower adjusted odds ratios of dose escalation at the 20% threshold () than patients treated with other IL-23, IL-12/23, TNF, and IL-17 inhibitors (all p < 0.0001).
Figure 5. Proportion of patients with ≥2 escalated dosing intervals above the 20% threshold during the maintenance period by (A) MOA and (B) biologic. *p < 0.05, **p < 0.01, ***p < 0.0001 compared to risankizumab based on chi-square test. IL: interleukin; MOA: mechanism of action; TNF: tumor necrosis factor. TNF inhibitor cohort includes adalimumab, etanercept, and certolizumab; IL-12/23 inhibitor cohort includes ustekinumab; IL-17 inhibitors cohort includes secukinumab, ixekizumab, brodalumab; other IL-23 inhibitors cohort includes guselkumab and tildrakizumab.
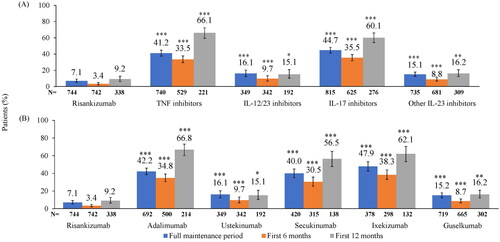
At the individual biologic level, the percentage of patients with dose escalation was significantly lower with risankizumab (7.1%) than with guselkumab, ustekinumab, secukinumab, adalimumab, and ixekizumab (15.2%, 16.1%, 40.0%, 42.2%, and 47.9%, respectively) at the 20% threshold (all p < 0.0001; ). Results were similar for the first 6 and 12 months of the maintenance period (all p < 0.0001 for the first 6 months and all p < 0.05 for the first 12 months). Patients in the risankizumab cohort had significantly lower adjusted odds ratios of dose escalation at the 20% threshold () than patients treated with guselkumab, ustekinumab, secukinumab, adalimumab, and ixekizumab (all p < 0.0001).
Discussion
This real-world study of patients with moderate-to-severe PsO contributes important and novel findings to the research on dose escalation of newly approved therapies for PsO such as risankizumab and compares it to other well-established therapies. Our findings demonstrate that a significantly lower percentage of risankizumab-treated patients had dose escalations compared with those receiving other biologics at the MOA level (TNF, IL-12/23, other IL-23, or IL-17 inhibitors) and individual biologic level (adalimumab, ustekinumab, guselkumab, secukinumab, or ixekizumab).
The results of this study support previously published data on the extent to which dose escalation is used in real-world practice (Citation7,Citation8), as well as the high variation observed between dose escalation rates of biologics in this study (Citation9). These high rates of dose escalation suggest that an inadequate response or dissatisfaction with the dosing regimen or biologic is common among patients with PsO, such that dose escalation is considered a reasonable option to improve the response to the biologic (Citation11,Citation30).
Studies of efficacy demonstrate that approximately 30% of patients receiving biologics for treatment of PsO do not achieve PASI 75 response, 20–32% lost PASI 75 response over 0.8–3.2 years, and 20% are dissatisfied with treatment (Citation5,Citation31,Citation32). For patients not reaching treatment goals, the options available include dose escalation, adding a topical agent for patients who have not achieved complete clearance, or switching to another biologic (Citation33). All 3 options may incur added costs and may not provide the desired outcome (Citation33). Dose escalations have been associated with increased costs incurred due to an increase in the amount of drug taken (Citation9,Citation11,Citation33). Therefore, there is an unmet need for a PsO treatment that is effective in achieving and maintaining treatment goals.
Dose-escalated use of biologics is associated with higher PsO-related costs than their labeled use, resulting in increased annual medical and pharmacy costs (Citation11,Citation34). In other real-world research, patients receiving above-label doses of biologics had higher PsO-related medical costs than those receiving on-label doses during the maintenance period ($673–$984 vs $549–$706) (Citation11). Additionally, PsO-related pharmacy costs were greater in patients receiving above-label dosing compared with those receiving on-label doses ($49,768–$54,790 vs $34,816–$49,588) (Citation11). Therefore, management of PsO is more costly for patients when treatment requires dose escalation to manage disease.
Risankizumab has demonstrated its reliability as a 12-week dose maintenance treatment in maintaining skin clearance in patients with moderate-to-severe PsO when compared with placebo, and sustained efficacy at 3 and 4.5 years of continuous use (Citation17,Citation18,Citation35). Therefore, risankizumab not only is an effective and durable treatment option, but also may be more predictable with regard to cost management due to reliable dosing for patients with moderate-to-severe PsO.
Long-term research showed that dose escalations were more frequent at 5 years versus 1 year of treatment for adalimumab, etanercept, and ustekinumab, suggesting that the longer patients with moderate-to-severe PsO take specific biologics, the more likely it is that they will receive dose escalations (Citation9). Therefore, findings from this study are encouraging in that they demonstrate significantly lower proportions of patients increasing dose over time with risankizumab compared with drugs with other MOAs and other biologics. However, long-term studies of risankizumab compared with other biologics are needed to confirm these findings.
Strengths of this study include that no prior research has looked at dose escalation encompassing all biologics approved by the FDA in this study time period, including recently approved IL-23 inhibitors. Additionally, this study utilized a large geographically and socioeconomically diverse commercial insurance database to describe observed dose escalation, providing robust and reliable data. A limitation of this analysis is that it presents claims data used in billing health plans and may be subject to data errors (e.g., miscoding). Additionally, the presence of a claim as well as the drug quantity for a filled prescription may not indicate actual use of the drug by a patient. Sampling is not captured in payer claims databases; thus, for some products dose escalation may have been underestimated. Furthermore, most of these medications are self-administered and may over- or under-estimate the frequency of dose escalation.
The results of this real-world study demonstrated that variability exists in dose escalation across biologics used to treat moderate-to-severe PsO during the maintenance treatment period. Risankizumab-treated patients with PsO demonstrated reliable dosing and had a significantly lower proportion of dose escalations compared with patients treated with other biologics.
Author contributions
AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. All authors had access to the data results and participated in the development, review, and approval of this manuscript.
Acknowledgments
Medical writing services provided by Natalie Mitchell, of Fishawack Facilitate Ltd, part of Fishawack Health, and funded by AbbVie.
Disclosure statement
Jashin Wu is or has been an investigator for AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Pfizer; a consultant for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health; and a speaker for AbbVie, Amgen, Bausch Health, Novartis, Regeneron, Sanofi Genzyme, Sun Pharmaceutical, and UCB.
Feng Zeng was a paid employee of Tigermed-BDM, which is a paid consultant to AbbVie, when she worked on this project. She is currently employed by Shionogi Inc, Florham Park, NJ, USA.
Ahong Huang, Xing Pan, and Yiwen Cao are paid employees of Tigermed-BDM, which is a paid consultant to AbbVie.
Manish Patel, Naijun Chen, Huzefa Photowala, and Vishvas Garg are full-time salaried employees of AbbVie and may own stock/options.
Jeff Crowley has received research/grant support from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, Lilly, MC2 Therapeutics, Merck, Novartis, Pfizer, Regeneron, Sandoz, Sanofi, Sun Pharma, UCB, and Verrica. He has served as a consultant for AbbVie, Amgen, BMS, Celgene, Dermira, Lilly, Novartis, Sun Pharma, and UCB; and has worked on speaker bureaus for AbbVie, BMI, Boehringer Ingelheim, Janssen, Lilly, Novartis, Regeneron, Sanofi, and UCB.
Data availability statement
The data that support the findings of this study are available from Merative® MarketScan® Research. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author upon request with the permission from Merative® MarketScan® Research.
Additional information
Funding
References
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1–9.
- Papp KA, Gniadecki R, Beecker J, et al. Psoriasis prevalence and severity by expert elicitation. Dermatol Ther. 2021;11(3):1053–1064.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 national health and nutrition examination surveys. J Am Acad Dermatol. 2021;84(3):767–769.
- Levin EC, Gupta R, Brown G, et al. Biologic fatigue in psoriasis. J Dermatolog Treat. 2014;25(1):78–82.
- Leonardi CL, See K, Burge R, et al. Number needed to treat network meta-analysis to compare biologic drugs for moderate-to-severe psoriasis. Adv Ther. 2022;39(5):2256–2269.
- Carrascosa J-M, Garcia-Doval I, Pérez-Zafrilla B, et al. Use of off-label doses is frequent in biologic therapy for moderate to severe psoriasis: a cross-sectional study in clinical practice. J Dermatolog Treat. 2015;26(6):502–506.
- Esposito M, Gisondi P, Conti A, et al. Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol. 2017;31(5):863–869.
- Gambardella A, Licata G, Sohrt A. Dose adjustment of biologic treatments for moderate-to-severe plaque psoriasis in the real world: a systematic review. Dermatol Ther. 2021;11(4):1141–1156.
- Pinter A, et al. Dose escalation of biologic treatment in patients with moderate-to-severe psoriasis in Japan P1606 Poster presented at: European Academy of Dermatology and Venereology Congress; September 2022, Milan, Italy.
- Bagel J, Glick B, Wu JJ, et al. Dose escalation and associated costs in biologic treatment of psoriasis based on real-world data. J Med Econ. 2021;24(1):782–791.
- Iskandar I, Ashcroft D, Warren R, et al. Patterns of biologic therapy use in the management of psoriasis: cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol. 2017;176(5):1297–1307.
- Gniadecki R, Leonardi C, Gordon K, et al. Long‐term optimization of outcomes with flexible adalimumab dosing in patients with moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32(8):1297–1304.
- van den Reek JM, van Lümig PP, Kievit W, et al. Effectiveness of adalimumab dose escalation, combination therapy of adalimumab with methotrexate, or both in patients with psoriasis in daily practice. J Dermatolog Treat. 2013;24(5):361–368.
- AbbVie. AbbVie expands immunology portfolio in the U.S. with FDA approval of SKYRIZI™ (risankizumab-rzaa) for moderate to severe plaque psoriasis. [PressRelease]. 2019. [cited 2022 Mar 25]. https://news.abbvie.com/news/press-releases/abbvie-expands-immunology-portfolio-in-us-with-fda-approval-skyrizi-risankizumab-rzaa-for-moderate-to-severe-plaque-psoriasis.htm.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658.
- Papp KA, Lebwohl MG, Puig L, et al. Long‐term efficacy and safety of risankizumab for the treatment of moderate‐to‐severe plaque psoriasis: interim analysis of the LIMMitless open‐label extension trial beyond 3 years of follow‐up. Br J Dermatol. 2021;185(6):1135–1145.
- Ehrenberg R, Griffith J, Theigs C, et al. Dose escalation assessment among targeted immunomodulators in the management of inflammatory bowel disease. J Manag Care Spec Pharm. 2020;26(6):758–765.
- Cosentyx (secukinumab) injection, for subcutaneous use. Novartis. January 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125504s001s002lbl.pdf. Accessed on October 10, 2022.
- Taltz (ixekizumab) injection, for subcutaneous use. Eli Lilly. March 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125521s000lbl.pdf. Accessed on October 10, 2022.
- Siliq (brodalumab) injection, for subcutaneous use. Valeant Pharmaceuticals. February 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf. Accessed on October 10, 2022.
- Tremfya (guselkumab) injections, for subcutaneous use. Janssen Biotech. July 2020. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TREMFYA-pi.pdf. Accessed on October 10, 2022.
- Ilumya (tildrakizumab-asmn) injection, for subcutaneous use. Sun Pharma. March 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761067s000lbl.pdf. Accessed on October 10, 2022.
- Skyrizi (risankizumab-rzaa) injection, for subcutaneous use. AbbVie. April 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761105s000lbl.pdf. Accessed on October 10, 2022.
- Humira (adalimumab) injection, for subcutaneous use. AbbVie. February 2021. https://www.rxabbvie.com/pdf/humira.pdf. Accessed on October 10, 2022.
- Stelara (ustekinumab) injection, for subcutaneous or intravenous use. Janssen Biotech. August 2022. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf. Accessed on October 10, 2022.
- Cimzia (certolizumab pegol) for injection, for subcutaneous use. UCB. September 2019. https://www.cimzia.com/themes/custom/cimzia/docs/CIMZIA_full_prescribing_information.pdf. Accessed on October 10, 2022.
- Enbrel (etanercept) injection, for subcutaneous use. Pfizer. December 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103795s5503lbl.pdf. Accessed on October 10, 2022.
- Feldman SR, Zhao Y, Navaratnam P, et al. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(3):201–209.
- Armstrong A, Jarvis S, Boehncke W-H, et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: results of the clear about psoriasis worldwide survey. J Eur Acad Dermatol Venereol. 2018;32(12):2200–2207.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: A meta-analysis. JAMA Dermatol. 2020;156(3):258–269.
- Haidari W, Pona A, Feldman SR. Management of residual psoriasis in patients on biologic treatment. J Drugs Dermatol. 2020;19(2):188–194.
- Feldman SR, Zhao Y, Zhou H, et al. Economic impact of above-label dosing with etanercept, adalimumab, or ustekinumab in patients with psoriasis. J Manag Care Spec Pharm. 2017;23(5):583–589.
- AbbVie (2022). Durable efficacy in psoriasis achieved by SKYRIZI patients through to 4.5 years. https://www.abbviepro.com/gb/en/immunology/dermatology/products/skyrizi/efficacy.html. Accessed on August 25, 2022.
Appendix A
Figure A1. Patient attrition. AS: ankylosing spondylitis; CD: Crohn’s disease; HS: hidradenitis suppurativa; PsA: psoriatic arthritis; PsO: psoriatic arthritis; RA: rheumatoid arthritis; UC: ulcerative colitis; UV: uveitis. aThe date of the first biologic claim was considered the induction period index date and the type of the biologics was defined as the index biologic drug; patients with evidence of difference types of biologics use on the induction period index date were excluded. bThe start of the maintenance period was from the induction period index date + induction period + 7 grace days. cThe setting (inpatient/outpatient) of the diagnosis claim does not matter. d6 months prior to the induction period index date including the induction period index date. ePatients had no prior claim of the index biologic in the period starting from the earliest available data to the induction index date.
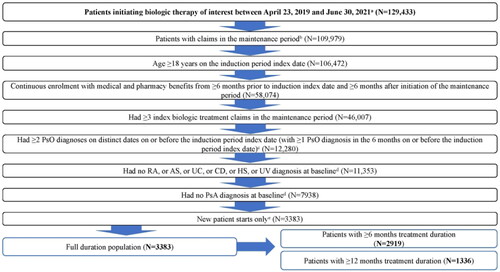
Table A1. Expected daily dosing in maintenance period.
Table A2. Baseline demographics and characteristics at biologic level.
Table A3. Average number of dose escalated claims and dose magnitude above the 30% threshold during the maintenance period by biologic.