Abstract
Background
Switching therapies is common for patients with psoriasis.
Objective
To quantify real-world switching rates and characteristics among patients initiating biologics over 24 months.
Methods
Patients aged ≥18 years with ≥2 confirmed psoriasis diagnoses who initiated a new biologic were identified from a US-payer claims database (Merative® MarketScan®) Switching rates were reported over 24 months using Kaplan–Meier survival analysis, and multivariable Cox regression analyses were performed to identify associated patient characteristics.
Results
A total of 7997 patients were included, with overall treatment switch rates at 14.4% at 12 months and 26.0% at 24 months. IL-23 inhibitors were associated with the lowest risk of switching compared with TNF, IL-17, and IL-12/23 inhibitors over 24 months (p < 0.0001). Switch rates varied between specific biologics, with the lowest switch rates observed for patients treated with risankizumab at 8.5% followed by guselkumab at 15.7% over 24 months. Prior targeted immune modulator use, age, and female gender were predictors of switching (adjusted hazard ratio; 1.23, 1.31, and 1.40, respectively; p ≤ 0.0005).
Limitations
Claims data may be subject to data errors and reasons for switching cannot be determined.
Conclusion
Switching was common in psoriasis patients using biologics over 24 months, with the lowest risk of switching observed with IL-23 inhibitors.
Introduction
Psoriasis is a chronic, inflammatory disease with a range of symptoms affecting the skin and joints. In the United States (US), prevalence of psoriasis is estimated at 3%, corresponding to approximately 7.5 million adults in 2020 (Citation1).
Common treatments for moderate-to-severe psoriasis include systemic medication (e.g., corticosteroids or methotrexate), small-molecule drugs (including apremilast), biologic therapies, and phototherapy (Citation2–5). The treatment landscape for psoriasis is evolving and recent advances in treatments mean that patients have access to newer biologics including therapies with activity against Interleukin (IL)-17 and IL-23 which may make achieving more stringent measures of disease control possible for more patients. Currently, there are four classes of biologics used to treat psoriasis: tumor necrosis factor (TNF) inhibitors (including infliximab (Citation6), etanercept (Citation7), adalimumab (Citation8), certolizumab pegol (Citation9)), IL-12/23 inhibitor (ustekinumab (Citation10)), IL-17 inhibitors (e.g., secukinumab (Citation11), ixekizumab (Citation12), brodalumab (Citation13)), and IL-23 inhibitors (guselkumab (Citation14), risankizumab (Citation15), tildrakizumab (Citation16)).
Switching therapies is common, and recommended strategy by the American Academy of Dermatology (AAD), for patients with psoriasis who experience a lack of treatment efficacy or safety/tolerability issues (Citation3,Citation17). The National Psoriasis Foundation has defined treat-to-target goals via expert consensus, including targeting for higher skin clearance to guide the evaluation of treatment response and adjustment of treatment and manage expectations for patients with psoriasis (Citation18). Recently, clinical trials have shown that switching to a biologic with a different mechanism of action can help achieve additional skin clearance and improvements in patient quality of life (QoL) (Citation19,Citation20). Identifying factors that influence switching may provide insights into how patients respond to and tolerate different treatments, which could help clinicians optimize disease management and allow patients to achieve higher treatment targets (e.g., Psoriasis Area Severity Index (PASI) 90 and 100) (Citation17,Citation18). Retrospective studies have evaluated adherence and persistence of advanced therapies in psoriasis; however, switching was often not a primary aim. Previous real-world analyses among patients with psoriasis estimate between 5–25% switch biologics within 1 year of initiation (Citation21,Citation22).
The objective of this study was to understand real-world switch rates among patients initiating biologics for psoriasis over 24 months using a large US-payer claims database, accounting for recent advancements in treatment during the study. Further aims included comparing switch rates by biological class, by individual biologic and understanding the demographic, clinical, and treatment characteristics associated with switching.
Methods
Study design and participants
This study was a retrospective cohort analysis utilizing data from 1 January 2016, to 31 March 2022 from The Merative® MarketScan® Commercial and Medicare Supplemental Research Databases with an early view. The database collected longitudinal, patient-level information on medical and pharmacy claims from a geographically diverse, large set of commercial electronic claims processors across the United States.
Eligible patients were aged 18 years and older who initiated a new treatment with a US Food and Drug Administration (FDA) approved biologic for psoriasis between 1 January 2018 and 30 September 2021 (index identification window with index date; defined as the date of treatment initiation) with ≥2 confirmed psoriasis diagnosis claims on separate days (including on index date) associated with a diagnostic code related to plaque/vulgaris psoriasis. Diagnostic codes included International Classification of Diseases (ICD)–9 (696.1; other psoriasis) and ICD-10 (L40.0 [psoriasis vulgaris], L40.8 [other psoriasis], L40.9 [psoriasis, unspecified]) codes. Patients had at least 6 months of continuous medical and pharmacy benefits coverage pre- and post-index date. Exclusion criteria included a diagnosis of any other biologic-indicated autoimmune conditions including psoriatic arthritis, hidradenitis suppurativa, uveitis, ulcerative colitis, Crohn’s disease, rheumatoid arthritis, ankylosing spondylitis, juvenile idiopathic arthritis in the 6 months pre- and post-index (Supplemental Figure 1).
Outcomes
This study evaluated switch rates and associated characteristics for psoriasis patients initiating biologics. Switch rate was defined as the proportion of patients who switched to a new targeted immune modulator ([TIM], biologic or apremilast) in the 24 months follow-up after the initiation of treatment. This study did not consider discontinuation or nonadherence in the switch definition. The proportion of patients switching to another biologic was quantified by overall cohort, by index biologic class (IL-23 inhibitors [guselkumab, risankizumab, and tildrakizumab], IL-12/23 inhibitor [ustekinumab], IL-17 inhibitors [brodalumab, ixekizumab, and secukinumab], and TNF inhibitors [adalimumab, certolizumab, etanercept, and infliximab]), and by individual biologic (only among cohorts with sufficient sample size [N > 100]).
Covariates
Baseline and demographic information were obtained on the index date, including geographic region, sex, age (grouped into aged 18–34,35–50,51–64, and 65 years and greater). Baseline clinical comorbidities, including anxiety or depression, major adverse cardiovascular events (myocardial infarction, stroke, and heart failure), hypertension, obesity and diabetes, were measured during the 6 months pre-index period (i.e. baseline). Baseline information on whether patients saw a dermatologist or a rheumatologist 6 months prior to or on the index date was also collected. In addition, the historical use of non-index biologic treatments or apremilast was identified prior to the index date of 1 January 2018.
Statistical analyses
Means and standard deviations (SDs) were calculated for continuous measures of demographic, clinical, and treatment characteristics of the cohort and categorical measures were reported as counts and percentages. The switch rate and first switch category were reported using descriptive statistics (percent and means). Kaplan–Meier survival analysis was used to estimate the proportion of patients remaining on index biologic (proportion of patients who did not switch to a new biologic/apremilast) over 24 months censoring for loss of follow-up due to end of enrollment. A log-rank test was used to calculate the P value to compare Kaplan–Meier curves of different cohorts and switch rates were calculated as percentages (100% – non-switch rate). Analyses were also performed using 12- and 24-months fixed follow-up periods (among patients with at least 12- and 24-months post-index continuous enrollment, respectively) for biologics to test the robustness of results compared to the Kaplan–Meier approach which accounts for censoring due to loss of follow-up. All comparisons between cohorts at the biologic class and individual biologic level used IL-23 inhibitors and risankizumab as the reference cohort, respectively.
Multivariable Cox (for Kaplan–Meier) and logistic (for fixed follow-up) regression analyses were performed to assess the impact of baseline demographics on switch rates using demographic (geographic region, sex, age), baseline dermatologist or rheumatologist visits, clinical (anxiety/depression, major adverse cardiovascular event, hypertension, obesity, diabetes) and treatment characteristics (index biologic as well as historical use of non-index biologics or apremilast) as covariates; adjusted hazard/odds ratios (HR/ORs) with 95% confidence intervals (CIs) were reported.
Data cleaning and preparation of the analytic dataset were conducted using the Instant Health Data (IHD) platform (Panalgo, Boston, MA, USA). Statistical analyses were also performed using the R packages installed in the IHD platform.
Ethical requirements
Data provided for the study were de-identified and Health Insurance Portability and Accountability Act compliant, therefore review board approval was not required.
Results
Study population
A total of 7997 patients with psoriasis were included in the analysis, comprising patients receiving IL-23 inhibitors (n = 2886; 36.1%), IL-17 inhibitors (n = 1855; 23.2%), TNF inhibitors (n = 2286; 28.6%), and IL-12/23 inhibitor (n = 970; 12.1%). The mean age of all included patients was 44.9 years (SD 12.8), and 48.3% were female (). Baseline characteristics are also consistent with individual biologics (Supplemental Table 1).
Table 1. Baseline characteristics.
Psoriasis treatment switch rates
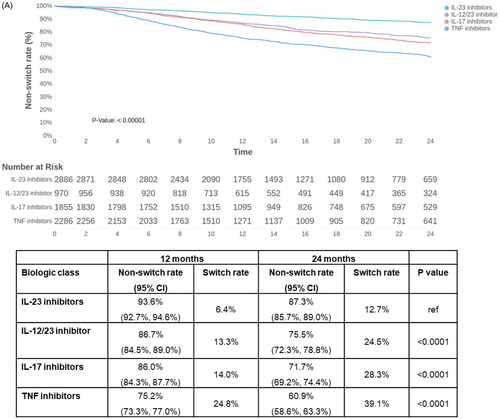
Across all biologics, the treatment switch rates were 14.4% at 12 months and 26.0% at 24 months (Supplemental Figure 2). Significant differences in switch rates were seen between biologic classes in the psoriasis population over 24 months (p < 0.0001). IL-23 inhibitors were associated with the lowest switch rates at 6.4% at 12 months and 12.7% at 24 months. The highest switch rates were seen with TNF inhibitors with observed switch rates of 24.8% and 39.1% at 12 months and 24 months, respectively ().
Figure 1. Switch rates over 24 months among biologics for patients with psoriasis [A] by biologic class, and [B] by individual biologic. Censoring is defined as the 24-month follow-up, first switch to apremilast/another biologic or end of continuous enrollment, whichever occurs first. CI: confidence interval; IL: interleukin; TNF: tumor necrosis factor.
![Figure 1. Switch rates over 24 months among biologics for patients with psoriasis [A] by biologic class, and [B] by individual biologic. Censoring is defined as the 24-month follow-up, first switch to apremilast/another biologic or end of continuous enrollment, whichever occurs first. CI: confidence interval; IL: interleukin; TNF: tumor necrosis factor.](/cms/asset/350d4def-da3c-4b5d-81db-b6038d2617ea/ijdt_a_2200870_f0001b_c.jpg)
Psoriasis treatment switching patterns by class of biologic
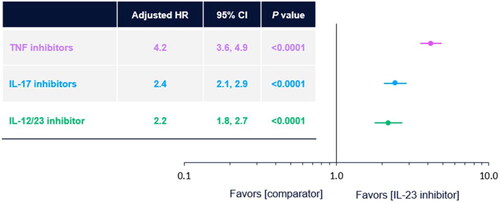
IL-23 inhibitors were also associated with the lowest risk of switching, after adjusting for baseline characteristics. Patients treated with TNF, IL-17, and IL-12/23 inhibitors were 4.2 (95% CI 3.6–4.9), 2.4 (95% CI 2.1–2.9), and 2.2 (95% CI 1.8–2.7) times more likely to switch than those treated with IL-23 inhibitors (p < 0.0001, based on multivariable Cox regression, ), respectively. Among those patients who switched biologic therapy, the majority switched to newer-generation biologics (35.2% switched to IL-17 inhibitors and 37.4% switched to IL-23 inhibitors, Supplemental Figure 3).
Figure 2. Adjusted risk of switching therapy by class of biologic. IL-23 inhibitor is the reference group. P value based on multivariable Cox regression models adjusting for baseline demographics (geographic region, gender, age), baseline provider type (dermatologist, rheumatologist, both, none), clinical comorbidities (anxiety/depression, major adverse cardiovascular event, hypertension, obesity, diabetes), and treatment characteristics (previous TIM use and index biologic class). CI: confidence interval; HR: hazard ratio; IL: interleukin; TIM: new targeted immune modulator; TNF: tumor necrosis factor.
Psoriasis treatment switch rates by individual biologic
Significant differences in switch rates were seen between specific biologics for patients with psoriasis (p < 0.0001). Over 12 months, the lowest switch rates were observed for risankizumab at 4.5% compared to all other biologics (p < 0.05) followed by guselkumab at 8.2%. Similarly, over 24 months, the lowest switch rates were observed for risankizumab at 8.5% compared to all other biologics (p < 0.05) followed by guselkumab at 15.7% (). The findings are consistent after adjusting for baseline characteristics (Supplemental Table 1), showing that risankizumab had the lowest switch rates relative to all included individual biologics (Supplemental Table 2).
Characteristics associated with switching
Prior TIM use, age (51–64 years), and female gender were important predictors of switching among psoriasis patients (adjusted HR = 1.2, 1.3, and 1.4, respectively; p ≤ 0.0005, ). Results were also consistent at the individual biologic level (p ≤ 0.0005, Supplemental Table 2).
Table 2. Predictors of switching biologic therapy: adjusted results from multivariable Cox regression analysis.
Switch rates in fixed follow-up periods
Similar results were seen in switch rates when evaluating fixed follow-up periods over 12 and 24 months for biologics. Switch rates increased over the 24-month period overall (Supplemental Figure 4 (A)) and across each drug class, with the lowest switch rates seen at both 12 and 24 months for IL-23 inhibitors and the highest seen for TNF inhibitors (Supplemental Figure 4 (B)). Similarly, switch rates for individual biologics increased from 12 to 24 months, with the lowest rates seen for risankizumab (compared to all other biologics; unadjusted and adjusted data; Supplemental Figure 4 (C)), followed by guselkumab.
Discussion
In this real-world retrospective study, switching was common among patients with psoriasis using biologics over 24 months, and switch rates were consistent with published studies that reported rates of 8–22% (Citation21–23). In the current study, significant differences in switch rates were identified between biologic classes, with IL-23 inhibitors demonstrating the lowest risk of switching over 24 months, and findings were consistent after adjusting for differences in baseline characteristics. At the individual biologic level, risankizumab had the lowest switch rates followed by guselkumab. IL-23 inhibitors and IL-17 inhibitors were also the classes of drugs to which patients were most likely to switch. Similar patterns have been previously reported, with patients receiving IL-23 inhibitors least likely to switch to another biologic and those most likely to switch to treated with TNF inhibitors (Citation24).
Recent studies with therapies targeting the IL-23 subunit -p19 (e.g., guselkumab and risankizumab) indicate efficacy in psoriasis and support their use as treatment options (Citation15,Citation25). Our study identified risankizumab with the lowest switch rate among individual biologics at both 12 and 24 months, followed by guselkumab, contributing to the current clinical evidence base that includes high rates of durable response through 172 weeks (over 3 years of continuous risankizumab treatment) (Citation26).
Results were consistent after adjusting for differences in baseline characteristics at both the mechanism of action and individual biologic levels as well as evaluating outcomes using fixed follow-up periods, demonstrating the robustness of this evidence.
When exploring predictors of switching, prior TIM use, age, and female gender were associated with switching among psoriasis patients. This is consistent with the current evidence base that has linked female gender, age under 65 years, and atherosclerotic conditions with a lower likelihood of adherence (Citation21). This previous study also demonstrated a potential association between the female gender and switching to a new biologic within 90 days of discontinuing the index biologic (Citation21).
Failure of initial biologic treatment may have a consequence of reduced patient satisfaction and lead to patient hesitation to try alternative biologics; therefore, it is important to explore challenges to switching therapy and consider patient-reported outcomes including QoL in treatment decisions (Citation21,Citation27). QoL assessment is flagged by the AAD as a consideration along with each patient’s clinical needs and treatment preferences before switching biologics and may be an area for future research (Citation3).
There is a high economic burden associated with managing psoriasis due to the complexities of the disease, including approximately half of the patients experiencing disruptions in therapy, such as switching biologic therapy or dose escalations, dose reductions, discontinuations, or restarts (Citation23). Selecting a first-line biologic is an important treatment decision, given that at 12 months, biologic-naïve patients with psoriasis who switched versus those who did not switch biologic incurred 20.3% higher monthly healthcare costs, largely driven by outpatient pharmacy costs (Citation28). In addition to considering clinical and cost-effectiveness evidence of benefits and harms of treatments, reviewing real-world treatment utilization patterns and associated healthcare costs may help inform US payers when reviewing their formularies (Citation29).
A strength of this study is that it adds to prior research examining treatment patterns by encompassing all biologics approved by the FDA in the study time period, including recently approved IL-23 inhibitors. This study utilized a large, geographically and socioeconomically diverse commercial insurance database to describe observed treatment patterns, providing robust and reliable data. This study is not without limitations, which include that the reasons for switching cannot be determined in claims data; therefore, no conclusions on efficacy or safety can be made. Claims data used for billing health plans were analyzed in this study and may be subject to data errors (e.g., miscoding). The presence of a claim for a filled prescription may not indicate the actual use of the drug by a patient. This retrospective, observational analysis utilized a large US-based administrative claims database that lacks important clinical details, such as disease severity and symptoms, and other patient socioeconomic and physician-specific information that may influence treatment choice. Despite modeling approaches to control for differences in characteristics available in administrative claims, it may be difficult to control for all potentially confounding variables which may impact observed switch rates.
In conclusion, this real-world study demonstrates that switching among patients with psoriasis using biologics was common over 24 months across a large US cohort. Significant differences existed in switch rates based on the class of biologic with IL-23 inhibitors demonstrated the lowest risk of switching over 24 months. In addition, risankizumab was associated with the lowest switch rates relative to all other biologics, followed by guselkumab. Important predictors of switching among psoriasis patients were identified as prior TIM use, age, and female gender, which adds to the current evidence on treatment switch patterns to help inform treatment decisions.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
| Abbreviations | ||
| AAD | = | American Academy of Dermatology |
| CE | = | continuous enrollment |
| CI | = | confidence interval |
| FDA | = | Food and Drug Administration |
| HR | = | hazard ratio |
| IL | = | interleukin |
| OR | = | odds ratio |
| PASI | = | Psoriasis Area Severity Index |
| PsA | = | psoriatic arthritis |
| PsO | = | psoriasis |
| QoL | = | quality of life |
| SD | = | standard deviation |
| TIM | = | targeted immune modulator |
| TNF | = | tumor necrosis factor |
| US | = | United States |
Supplemental Material
Download PDF (335.5 KB)Acknowledgments
Medical writing assistance was provided by Sarah Hodgkinson PhD, of Fishawack Facilitate Ltd, part of Fishawack Health, and was funded by AbbVie Inc., North Chicago, IL.
Disclosure statement
AW Armstrong has served as a research investigator and/or scientific advisor to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, BI, BMS, Dermavant, Dermira, EPI, Incyte, Leo, UCB, Janssen, Lilly, Nimbus, Novartis, Ortho Dermatologics, Sun, Sanofi, Regeneron, Pfizer, and Modmed. M Patel, C Li, V Garg, and MR Mandava are employees of AbbVie and may own AbbVie stock. JJ Wu is or has been an investigator, consultant, and/or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, DermTech, Dr. Reddy’s Laboratories, Eli Lilly, EPI Health, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Pfizer, Regeneron, Samsung Bioepis, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health.
Data availability statement
The data that support the findings of this study are available from Merative® MarketScan® Research. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author upon request with permission from Merative® MarketScan® Research.
Additional information
Funding
References
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):1–8.
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072.
- Menter A, Gelfand JM, Connor C, et al. Joint American academy of dermatology-national psoriasis foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486.
- Thatiparthi A, Martin A, Liu J, et al. Biologic treatment algorithms for moderate-to-severe psoriasis with comorbid conditions and special populations: a review. Am J Clin Dermatol. 2021;22(4):425–442.
- Chaudhari U, Romano P, Mulcahy LD, et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–1847.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022.
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115.
- Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302–314.e306.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–1674.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168(2):412–421.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286.
- Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–1040.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Papp K, Thaçi D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939.
- Kerdel F, Zaiac M. An evolution in switching therapy for psoriasis patients who fail to meet treatment goals. Dermatol Ther. 2015;28(6):390–403.
- Armstrong AW, Siegel MP, Bagel J, et al. From the medical board of the national psoriasis foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–298.
- Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37.
- Strober B, Armstrong A, Rubant S, et al. Switching to risankizumab from ustekinumab or adalimumab in plaque psoriasis patients improves PASI and DLQI outcomes for sub-optimal responders. J Dermatolog Treat. 2022;33(7):2991–2996.
- Doshi JA, Takeshita J, Pinto L, et al. Biologic therapy adherence, discontinuation, switching, and restarting among patients with psoriasis in the US medicare population. J Am Acad Dermatol. 2016;74(6):1057–1065.e1054.
- Foster SA, Zhu B, Guo J, et al. Patient characteristics, health care resource utilization, and costs associated with treatment-regimen failure with biologics in the treatment of psoriasis. J Manag Care Spec Pharm. 2016;22(4):396–405.
- Feldman SR, Zhao Y, Navaratnam P, et al. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(3):201–209.
- Gooderham MJ, Lynde C, Turchin I, et al. Real-world, long-term treatment patterns of commonly used biologics in Canadian patients with moderate-to-severe chronic plaque psoriasis. J Dermatol. 2022;49(1):95–105.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431.
- Gooderham M, Pinter A, Ferris LK, et al. Long-term, durable, absolute psoriasis area and severity index and health-related quality of life improvements with risankizumab treatment: a post hoc integrated analysis of patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(6):855–865.
- Norlin JM, Steen Carlsson K, Persson U, et al. Switch to biological agent in psoriasis significantly improved clinical and patient-reported outcomes in real-world practice. Dermatology. 2012;225(4):326–332.
- Wu JJ, Pelletier C, Ung B, et al. Real-world switch patterns and healthcare costs in biologic-naive psoriasis patients initiating apremilast or biologics. J Comp Eff Res. 2020;9(11):767–779.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513.