Abstract
Background
Mohs micrographic surgery (MMS) is the preferable surgery for difficult -to-treat basal cell carcinoma (BCC) but is an expensive, labor-intensive, and time-consuming technique. The aim of this study is to compare the efficacy and safety of photodynamic therapy combined with surgery(S-PDT) versus Mohs micrographic surgery (MMS) for the treatment of difficult-to-treat BCC.
Methods
This was a retrospective, comparative study. A total of 32 patients, 16 patients with 48 lesions, were treated with S-PDT, and the other 16 patients with 17 lesions treated by MMS were enrolled in this study. Follow-up was at least 36 months posttreatment.
Results
The recurrence rate was no statistical difference between the S-PDT and MMS (p = 1.000, Fishers exact test). The median follow-up was 42.5 months (range 36–63 months). The mean healing time in the S-PDT [17.9 d (SD 9.8)] is longer than in MMS [7.5 d (SD 1.5)] during follow-up (p<.001, Independent T-test). On the whole, the cosmetic outcome of patients in S-PDT was statistically no significant difference with that in MMS according to a 4-point scale (p = .719, chi-squared test).
Conclusions
S-PDT is a safe, effective, and novel cosmetic treatment, which holds the potential to be an alternative treatment to MMS for some cases.
Introduction
Basal cell carcinoma (BCC) is a prevalent malignancy among white populations worldwide (Citation1). In the United Kingdom, the European age-standardized incidence rates (EASRs) of the first BCC cases in 2013–2015 were recorded at 285 per 100,000 person-years, with a steady increase in incidence (Citation2,Citation3). This increase has contributed significantly to the global healthcare system’s burden (Citation4,Citation5), with estimated annual spending on keratinocyte skin cancer treatment ranging from £889 to £1226 in England in 2008 (Citation6,Citation7). Current estimates suggest that this spending has considerably increased due to the rising incidence, improved diagnosis, and treatment (Citation1,Citation3–6). Although BCC rarely metastasizes, it can be medically problematic due to its tendency to appear on the head and neck, particularly in the H-risk zone, leading to functional and esthetic issues (Citation1–4,Citation8).
Difficult-to-treat BCC (Citation4), include all locally advanced BCC and common BCC that are large lesions, located in functional areas, multiple lesions and belong to a high-risk BCC subtype or are recurrent. The incidence of difficult-to-treat BCC has been increasing in some countries (Citation9). Current treatments for difficult-to-treat BCC are MMS, radiotherapy, photodynamic therapy(PDT), chemotherapy, immunotherapy, and Hedgehog inhibition(Hh inhibitors)(vismodegib and sonidegib) (Citation10). Mohs Micrographic Surgery (MMS) is a widely used and effective treatment option for difficult-to-treat BCC, owing to its meticulous microscopic margin control that enables thorough tumor removal (Citation5). However, this technique is associated with significant drawbacks, including high costs, time consumption, and labor intensity (Citation11). One of the reason is that each frozen section has to be histologically analyzed to ensure complete tumor removal (Citation12). Ideally, the treatment for difficult-to-treat BCC should be carefully planned by a skin cancer multidisciplinary board, to choose the appropriate therapeutic schedule in specific cases.
PDT is a noninvasive therapeutic modality that is used for the treatment of BCC. It involves the use of specific wavelengths of light to irradiate tumors with a photosensitizing agent, which then undergoes photochemical reactions, resulting in selective tumor destruction (Citation4,Citation13). In the context of BCC treatment, PDT is typically prescribed for non-aggressive, low-risk BCC cases (Citation4). However, PDT has limited effectiveness for nodular BCC in certain countries, owing to the shallowness of its effective depth (ranging from 2-4mm) (Citation4,Citation13–16). In recent years, numerous studies have proposed that pretreatment can enhance PDT efficacy by promoting improved drug penetration and PpIX metabolization (Citation17–19).
The objective of this study was to evaluate and compare the effectiveness and safety of the combined approach of PDT combined with surgery (S-PDT) with MMS in managing difficult-to-treat basal cell carcinoma.
Materials and methods
Study design
In this retrospective study, the electronic medical records (EMR) at the Department of Dermatology, Xiangya Hospital, Central South University and Institute of Dermatology, Chinese Academy of Medical Science & Peking Union Medical College were searched to find all patients diagnosed with difficult-to-treat BCC between November 2015 to August 2018.
Patients with difficult-to-treat BCC were defined according to the European consensus-based guidelines of BCC in 2019:(1) the technical difficulty of maintaining function and esthetics due to the size or location (H zone) of the tumor; (2) poorly defined borders often associated with morphoeic subtype or prior recurrence; (3) multiple prior recurrences on the face (often requiring much larger excision); (4) patient characteristics(comorbidities, anticoagulation, pacemaker, advanced age and patient’s reluctance to accept the consequences of surgery) (Citation4,Citation20). The exclusion criteria included age below 18 years, a photosensitizer or lidocaine allergy, pregnancy or lactation, and any active systemic infectious diseases. Individuals also were excluded who had received treatment for the lesion within the last 30 d.
The treatment of difficult to BCCs were discussed at our Tumor Board (a skin cancer multidisciplinary team). The tumor board included experts from dermatology, oncology, surgery, and pathology, and they recommended S-PDT for cases where MMS was not feasible due to various reasons such as tumor size, location, comorbidities, pacemaker placement, advanced age, or patient preference. The study enrolled two groups of patients who underwent either S-PDT or MMS according to the same treatment protocol in two different hospitals. The retrospective trial adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Xiangya Hospital of Central South University and Institute of Dermatology, Chinese Academy of Medical Science, and Peking Union Medical College. Informed consent was obtained from all participants, and a minimum follow-up of 36 months was conducted. A comprehensive review of all patient records was completed in November 2018.
Treatment procedure
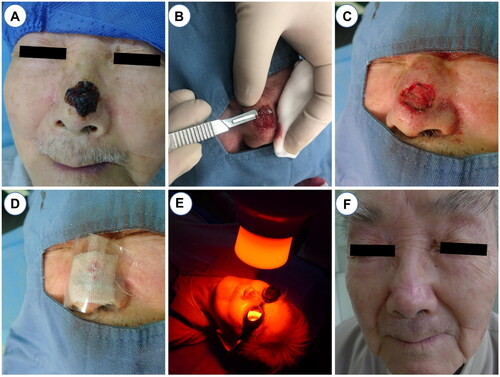
Prior to the utilization of S-PDT, tumor boundaries were identified using a dermoscope (Fotofinder 1000). Standard disinfection protocols, towel placement, and local anesthesia with 1% lidocaine were carried out prior to surgical preparation. For most lesions, in-situ resection was performed along the boundaries of the dermoscope, followed by simple suturing (Figure S1). However, for lesions with high local tension, such as those located on the nasal region, superficial shaving was opted for to prevent complications from simple suturing. The shaving method involved the removal of the visible tumor using a razor blade (Gillette brand), with the depth of the papillare cutis to obtain punctate bleeding. Electrocoagulation was used to halt bleeding when necessary, ensuring complete removal of the tumor with the naked eye. In-situ excision or shaving was performed only once before the first PDT. A solution of 20% 5-aminolaevulinic acid (5-ALA) (Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd.®, Shanghai, China) was applied to the lesion and surrounding area 0.5 cm beneath a multilayer dressing. The patient was then instructed to avoid light in a dark environment for 3 h before irradiation with 633 nm red light at 80 m W/cm2 for 20 min. The ALA-PDT procedure was repeated a total of three times, with a one-week interval between each treatment ().
Figure 1. The procedures of S-PDT. (A) Routine disinfection towels and local lidocaine anesthesia. (B) Superficial shaving with a scalpel or razor. (C) Electrocoagulation was used to stop bleeding. (D)Apply pressure dressing with gauze, sterile gloves, cotton pads, or bandages. (E) Red light with wavelength of 633 nm and power of 80mW/cm2 was irradiated for 20 min. (F) Good postoperative recovery at 36 months follow-up.
The MMS procedures were conducted by dermatologic surgeons who possessed professional experience of more than five years. The tumor excision was performed at a 45-degree angle subsequent to local anesthesia, utilizing a lidocaine (1%) solution that contained epinephrine (0.001%). A bowel-shaped excision specimen was obtained by extending the resection margin by 2 mm and forwarded for histological margin examination (Citation21). The specimen was compressed and processed into horizontal frozen sections by a pathologist. In cases where a residual tumor was found in the specimen, the removal process was repeated until no residual tumor was detected. The decision for skin reconstructions, including closure with a local skin flap or graft, was made after taking into account the size of the defect, cosmetic effect, and expense, in consultation with the patients and the operator.
Outcomes measures
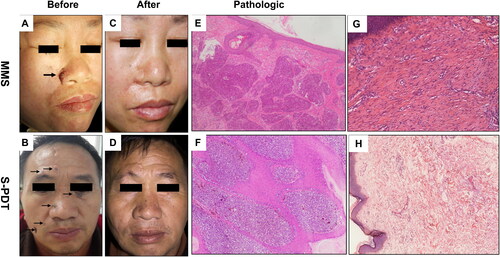
Medical records and photographs were the sources of EMR. We collected the baseline information, including gender, age, duration, location, size, and number of lesions. Diagnosis of BCC was set on a histological basis ().
Figure 2. Clinical and pathology pictures in MMS and S-PDT groups. (A,B) Clinical picture with one lesion before treatment and 12 mouth follow-up in MMS group. (C,D) Clinical picture with four lesions before treatment and 12 mouth follow-up in S-PDT group. (E,F) Pathology before treatment in both groups shows basal cell carcinomas with basal cell-like cell hyperplasia, cytoplasm, deep staining of the nucleus, nucleus, disordered arrangement (hematoxylin–eosin stain, original magnification ×100). (G) Pathological biopsies after 1 year of follow-up in MMS group and show scar tissue. (F) Pathological biopsies after 2 years of follow-up in S-PDT group and show scar tissue and no recurrent pathological features.
The primary endpoint was defined as the probability that a patient was free of tumor recurrence (complete healing of the tumor, without any elevation or infiltration and normal texture of the skin) at least 36 months follow-up. Patients were followed up for three years with remote video (Wechat) interviews and clinic visits at 12, 24, and 36 months. The evaluation of tumor recurrence was based on clinical symptoms, dermoscopy images, and pathological examination if necessary, as determined by the dermatologist (, Figure S1).
Secondary endpoints included mean healing time, dermatology, life quality index (DLQI), cosmetic outcomes, total treatment time, and cost. The end of healing time was determined by the dermatologist according to the patient’s recovery in the postoperation. The DLQI per person were evaluated before and after treatment, which used to indicate the improvement in patient quality of life.
The cosmetic outcome was assessed on a four-point scale on the base of the appearance of the tumor within 1 cm around the scar and the overall cosmetic appearance, determined by the patient: 0: poor/large, retractile or hypertrophic scar, 1: fair/visible, infiltrated scar, 2: good/scar without any distortion, thin, hardly visible, 3: excellent/almost invisible (Citation22).
From the surgeon’s perspective, the total treatment time was calculated, which included the surgical procedure and photodynamic irradiation time for S-PDT and preoperative preparation, surgical resection, intraoperative pathology, and skin reconstruction for MMS. However, other times such as postoperative recovery, 5-ALA cream incubation, and travel time were not included. The total cost of treatment was determined through a telephone or Wechat survey, which encompassed the entire healthcare cost incurred within a year, excluding expenses incurred outside the healthcare system such as travel and nutritional costs, which were minimal. The healthcare cost was determined based on the Unified Fees for medical services in China, and all costs are presented in euros.
Statistical analysis
Data are frequencies, percentages, and means ± standard deviation. Independent T and the chi-square (and Fisher’s exact) tests were used for statistical analysis wherever appropriate. As cost data generally have a skewed distribution, the Mann–Whitney tests are used for the cost analysis and data are presented as median. The significance level was p < .05. All analyses were carried out using IBM SPSS 25.
Results
Characteristics
A total of 39 patients were initially enrolled in the study, but 7 were excluded due to loss to follow-up immediately after treatment. Of the remaining 32 cases, 16 patients (5 women, 31.25%) with 48 lesions were treated with S-PDT (as detailed in Tables S1 and S2), and 16 patients (5 women, 31.25%) with 17 lesions were treated with MMS. The majority of the lesions were located in the H-zone (n = 64), with 47 treated using S-PDT and 17 with MMS. Tumor size was categorized as being less than or equal to 15 mm, or greater than 15 mm in diameter (Citation20). No significant differences were found between the two groups with regards to age, gender, duration, localization and number of lesions, histological type, and tumor size. These baseline characteristics are presented in . The median follow-up time was 47.8 months, with a range of 36–66 months.
Table 1. Baseline characteristics in the S-PDT and MMS group.
Primary outcome
A total of 48 lesions were treated with S-PDT, where 31 received superficial shaving pretreatment and 17 received in-situ excision pretreatment (Tables S1 and S2). Repeat biopsies have taken the center of lesions and were performed at the original biopsy site in some patients (8/16) approximately 12 months after S-PDT, and no recurrence was observed. Additionally, video interviews and clinic visits at 24 and 36 months confirmed no recurrence in any of the patients (Tables S1 and S2). Statistical analysis using Fisher’s exact test indicated that there was no significant difference in the recurrence rate between the S-PDT and MMS groups (p = 1.000, ). In the MMS group, one patient experienced NBCC in the right periocular area, which recurred after 5 months. Secondary surgery was performed by expanding a 2 mm resection based on the recurrent lesion. Follow-up repeat biopsies revealed no tumor recurrence at 24 months after secondary surgery.
Table 2. Therapeutic effects, DLQI and total treatment time.
Secondary outcomes
The mean healing time in the S-PDT group was 17.9 d (SD 9.8), which was longer than that in the MMS group (7.5 d, SD 1.5). However, there was no statistically significant difference in healing time between the two groups (estimated mean difference [MD] 5.04 d [95% CI 5.40–15.47]; p<.001; ).
Cosmetic outcomes were assessed using the patient-reported outcomes of 48 lesions treated with S-PDT and 17 lesions treated with MMS after 12 months. In the S-PDT group, 21 lesions (43.75%) showed excellent cosmetic results, 17 (35.42%) were assessed with good cosmetic results, 8 (16.67%) with fair cosmetic results, and 2 (4.16%) with poor cosmetic results. In the MMS group, four lesions (23.52%) presented excellent cosmetic results, 9 (52.94%) showed good cosmetic results, 3 (17.64%) with fair cosmetic results, and 1 (5.88%) with poor cosmetic results. The results showed no statistically significant difference between the two groups in terms of cosmetic outcomes (p = .719, chi-squared test) (Supplementary materials) ().
Table 3. Cosmetic response to S-PDT and MMS at 12 months after treatment.
Patient-reported outcomes for quality of life were assessed using the dermatology life quality index (DLQI). Before treatment, there was no statistically significant difference in DLQI scores between the S-PDT and MMS groups (estimated MD, 0.19 [95% CI −1.88 to 2.54]; p = .854; ). After treatment, the mean DLQI score in the S-PDT group was 0.88 (SD 1.67), which was lower than that in the MMS group (mean 3.44, SD 2.90) (estimated MD 2.56 [95% CI 0.86–4.27]; p = .016; ).
According to our analysis, we estimated the times to treatment for each individual participant in the study. Our findings revealed that the mean treatment time for the S-PDT group was 2.08 h (SD 0.65), which was slightly lower than the mean treatment time of 3.62 h (SD 1.07) for the MMS group. The estimated mean difference (MD) between the two groups was 1.55 h (95% CI 0.91–2.19), with a statistically significant difference observed (p < .001; ). In terms of costs, we calculated the per-person treated costs for each group. Our analysis demonstrated that the median and mean costs for S-PDT treatment were lower than those for MMS treatment (€621 vs €869 and €700 vs €1042, respectively). The Mann-Whitney test revealed a significant difference in costs between the two groups (p = .003, ).
Table 4. Total costs both in S-PDT and MMS group (Euro).
Adverse events
Adverse events (AEs) were monitored throughout the course of the study, including pain and other symptoms. The pain was evaluated using the visual analog scale (VAS), which ranges from no pain (score of 0) to unbearable pain (score of 10). The most common AEs associated with S-PDT were mild pain (31/48, 64.5%) and itching (18/48, 37.5%), followed by telangiectasia (8/48, 16.7%), burning sensation (5/48, 10.4%), erythema (3/48, 6.3%), and scabs (3/43, 6.3%) (Figures S2(D–F)). These AEs tended to subside over time. Most patients experienced pain during or after the procedure, with pain scores ranging from 0 to 4 on the VAS, indicating only mild pain. In contrast, visible hypertrophic scars were the most frequently reported AE in the MMS group (7/17, 41.1%), followed by skin redness and swelling (3/17, 17.6%) and skin infection (2/17, 11.7%) (Figures S2(A–C)). Pain scores in the MMS group were also below 4 on the VAS and did not differ significantly from those in the S-PDT group.
Discussion
The findings of this investigation indicate that S-PDT is associated with lower recurrence rates, superior cosmetic outcomes, reduced healthcare expenditures, and favorable postoperative DLQI scores. These outcomes suggest that S-PDT may be a viable option for patients with difficult-to-treat BCCs.
Difficult-to-treat BCC poses significant challenges for clinical management. Common treatments include MMS, radiotherapy, PDT, chemotherapy, immunotherapy, and Hedgehog (Hh) inhibition with vismodegib and sonidegib (Citation10).MMS with microscopic margin control is the most frequently used approach for difficult-to-treat BCC. While radiotherapy may be a good alternative to S-PDT for these patients, its potential side effects, including long-term atrophic disorders and neoplasia, make it unsuitable for elderly individuals (Citation4,Citation23). The combination of radiotherapy and surgery may lead to regional tissue necrosis and high treatment cost (Citation23,Citation24). Hh inhibitors, including vismodegib and sonidegib, are indicated for the treatment of locally advanced BCC in patients who are not suitable for surgery or radiotherapy (Citation4). Combinations of Hh inhibitors for difficult-to-treat BCC have also been reported (Citation25,Citation26). Given the refractory nature, high-risk, and tendency to relapse, multidisciplinary tumor board discussions are preferred for making treatment decisions.
PDT has been utilized as an effective treatment for actinic keratosis (AK), Bowen’s disease (BD), and certain nodular BCCs (NBCCs), with remarkable cosmetic outcomes. A decade ago, prospective, multicenter clinical studies indicated that PDT could also be employed for difficult-to-treat BCC with a complete response rate of 78% after 24 months (Citation20,Citation27). However, recent guidelines have highlighted that PDT should not be administered in high-risk tumor survival and deep penetration areas, particularly the facial ‘H’-zone (Citation4), due to the restricted effective depth of topical methyl aminolevulinate PDT and 5-aminolevulinic PDT. To ensure the efficacy of PDT, Marieke H et al. have suggested that pathologists measure the thickness of NBCCs before treatment (Citation28). Therefore, for thicker lesions, PDT combined with surgical pretreatment is recommended. Other pretreatments, such as CO2 laser, in-situ excision, superficial shaving, curettage, and multiple needles, may also reduce tumor thickness and enable adequate photodynamic therapy. Surgical pretreatment offers particular advantages in clinical practice, particularly in hard-to-operate areas, as superficial shaving and in-situ excision are relatively easy to master and require only about 10 min, resulting in reduced surgical burden for patients. Moreover, the required instruments, razor blades, and surgical blades are easily obtainable, facilitating the technique’s use in low-budget settings.
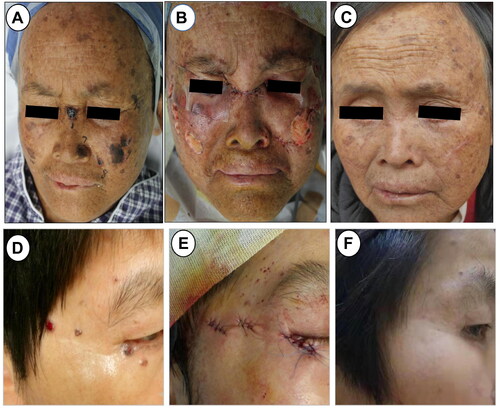
The utilization of surgery in conjunction with PDT should be restricted to specific cases, contingent upon deliberation by a local multidisciplinary tumor board. For instance, in our study, three patients from the S-PDT group exhibited a minimum of ten facial lesions each (). These cases pose a significant challenge for reconstruction subsequent to MMS and necessitate a prolonged process of anesthesia and pathological sectioning. Moreover, among the S-PDT group, there were three patients over 85 years of age, with potential inadequacy of cardiopulmonary function to withstand the MMS procedure. Interestingly, one patient refused MMS, and thus we opted for S-PDT treatment instead. In general, S-PDT was employed solely when MMS was unsuitable as determined by the tumor board, in instances of unconventional size or location, a high number of lesions, patient characteristics (comorbidities, pacemaker, and advanced age), and patient preference.
Figure 3. Clinical picture of two patients in S-PDT group. (A) Multiple basal cell carcinomas of the facial ministry in a 52-year-old woman. (B) Clinical photograph immediately after superficial shaving treatment. (C) Clinical photograph after a follow-up of 12 months. (D)Basal cell carcinoma of the right eye periphery in a 56-year-old woman. (E) Clinical photograph after simple excision and suturing. (F) Clinical photograph after a follow-up of 24 months.
In the present study, 79% of individuals in the S-PDT group achieved satisfactory cosmetic outcomes, which were comparable to those achieved by MMS (Figure S2). Numerous studies have demonstrated that MMS yields excellent cosmetic outcomes and high patient satisfaction. However, MMS is associated with several drawbacks. Firstly, the indications for MMS have been expanded in recent years, yet there is a shortage of qualified dermatologists capable of performing the procedure (Citation29). Secondly, training dermatologic surgeons to achieve optimal cosmetic outcomes through MMS is time-consuming. Thirdly, MMS is a costly treatment, regardless of the location where it is performed. If MMS were to be employed for all difficult-to-treat BCC indications (Citation30), this would result in a significant healthcare burden on both individuals and society. Lastly, evidence suggests that PDT may offer greater value for money than excision for BCCs. Furthermore, when the cost of surgical pretreatment is taken into account, the costs associated with S-PDT are lower than those of MMS (Citation31). Our investigation also confirmed that the average cost of S-PDT (€700) was considerably lower than that of MMS (€1042).
Limitations
Limitations of the study include the inadequate thickness data for NBCCs prior to S-PDT. The study assessed only four lesions, with a mean thickness of 3.02 mm and a maximum of 4.4 mm. Furthermore, the majority of lesions treated with S-PDT were located in the facial ‘H’-zone, which presented challenges for achieving adequate penetration of the PDT. Additionally, the sample size and follow-up duration were limited. To more comprehensively demonstrate the efficacy of S-PDT therapy, a larger randomized controlled trial with longer-term follow-up and increased patient enrollment will be necessary.
Conclusion
Our study’s results revealed the feasibility of combining PDT with surgery to treat difficult-to-treat BCCs. This approach could potentially serve as an alternative to MMS for certain cases, such as unconventional tumors in terms of location or size, multiple lesions, comorbidities in patients, patient refusal of MMS, or advanced age. Therefore, this combined treatment method may provide a viable option for selected patients with difficult-to-treat BCCs.
Ethical approval
The trial was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Xiangya Hospital of Central South University and institute of Dermatology, Chinese Academy of Medical Science & Peking Union Medical College.
Consent form
Not applicable.
Supplemental Material
Download PDF (371.8 KB)Acknowledgements
The patients in this manuscript have given written informed consent to publication of their case details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Work G, Invited R, Kim JYS, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78(3):1–8.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017;92(6):890–898.
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10(4):37–46.
- Peris K, Fargnoli M, Garbe C, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer. 2019;118:10–34.
- Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(5):574–597.
- Venables ZC, Nijsten T, Wong KF, et al. Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013–15: a cohort study. Br J Dermatol. 2019;181(3):474–482.
- Vallejo-Torres L, Morris S, Kinge J, et al. Measuring current and future cost of skin cancer in England. J Public Health. 2014;36(1):140–148.
- Adalsteinsson JA, Ratner D, Olafsdottir E, et al. Basal cell carcinoma: an emerging epidemic in women in Iceland. Br J Dermatol. 2020;183(5):847–856.
- Kappelin J, Green A, Ingvar Å, et al. Incidence and trends of basal cell carcinoma in Sweden: a population-based registry study. Br J Dermatol. 2022;186(6):963–969.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100(11):adv00140.
- Sebaratnam D, Choy B, Lee M, et al. Direct cost-analysis of Mohs micrographic surgery and traditional excision for basal cell carcinoma at initial margin clearance. Dermatol Surg. 2016;42(5):633–638.
- Walocko F, Chelliah P, Kolitz E, et al. Basal cell carcinoma histopathologic upgrading and Mohs micrographic surgery: a single institution, retrospective review. Arch Dermatol Res. 2022;314(7):705–707.
- Champeau M, Vignoud S, Mortier L, et al. Photodynamic therapy for skin cancer: how to enhance drug penetration? J Photochem Photobiol B. 2019;197:111544.
- Bay C, Lerche CM, Ferrick B, et al. Comparison of physical pretreatment regimens to enhance protoporphyrin IX uptake in photodynamic therapy: a randomized clinical trial. JAMA Dermatol. 2017;153(4):270–278.
- Lutfiyya MN, Chang LF, McGrath C, et al. The state of the science of interprofessional collaborative practice: a scoping review of the patient health-related outcomes based literature published between 2010 and 2018. PLOS One. 2019;14(6):e0218578.
- Yu N, Luo X, Wei T, et al. Dermabrasion combined with photodynamic therapy: a new option for the treatment of non-melanoma skin cancer. Lasers Med Sci. 2022;37(2):1255–1263.
- Zhao S, Liu D, Shi W, et al. Efficacy of a new therapeutic option for vulvar intraepithelial neoplasia: superficial shaving combined with photodynamic therapy. Lasers Surg Med. 2020;52(6):488–495.
- Bu W, Zhang M, Zhang Q, et al. Preliminary results of comparative study for subsequent photodynamic therapy versus secondary excision after primary excision for treating basal cell carcinoma. Photodiagnosis Photodyn Ther. 2017;17:134–137.
- Huang K, Xie Y, Li M, et al. A comparative study: superficial shaving combined with photodynamic therapy versus cryotherapy in the treatment of recalcitrant plantar warts. Lasers Surg Med. 2020;52(8):747–752.
- Vinciullo C, Elliott T, Francis D, et al. Photodynamic therapy with topical methyl aminolaevulinate for ‘difficult-to-treat’ basal cell carcinoma. Br J Dermatol. 2005;152(4):765–772.
- Mosterd K, Krekels G, Nieman F, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149–1156.
- Cosgarea R, Susan M, Crisan M, et al. Photodynamic therapy using topical 5-aminolaevulinic acid vs. surgery for basal cell carcinoma. J Eur Acad Dermatol Venereol. 2013;27(8):980–984.
- Visch Marjolein Birgitte M, Kreike Bas B, Gerritsen Marie-Jeanne Pieternel M. Long-term experience with radiotherapy for the treatment of non-melanoma skin cancer. J Dermatolog Treat. 2020;31(3):290–295.
- Cho M, Gordon L, Rembielak A, et al. Utility of radiotherapy for treatment of basal cell carcinoma: a review. Br J Dermatol. 2014;171(5):968–973.
- Pollom E, Bui T, Chang A, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151(9):998–1001.
- Jain R, Kumar Dubey S, Singhvi G. The hedgehog pathway and its inhibitors: emerging therapeutic approaches for basal cell carcinoma. Drug Discovery Today. 2022;27(4):1176–1183.
- Horn M, Wolf P, Wulf HC, et al. Topical methyl aminolaevulinate photodynamic therapy in patients with basal cell carcinoma prone to complications and poor cosmetic outcome with conventional treatment. Br J Dermatol. 2003;149(6):1242–1249.
- Roozeboom MH, Aardoom MA, Nelemans PJ, et al. Fractionated 5-aminolevulinic acid photodynamic therapy after partial debulking versus surgical excision for nodular basal cell carcinoma: a randomized controlled trial with at least 5-year follow-up. J Am Acad Dermatol. 2013;69(2):280–287.
- Blechman AB, Patterson JW, Russell MA. Application of Mohs micrographic surgery appropriate-use criteria to skin cancers at a university health system. J Am Acad Dermatol. 2014;71(1):29–35.
- Hoorens I, Batteauw A, Van Maele G, et al. Mohs micrographic surgery for basal cell carcinoma: evaluation of the indication criteria and predictive factors for extensive subclinical spread. Br J Dermatol. 2016;174(4):847–852.
- Caekelbergh K, Annemans L, Lambert J, et al. Economic evaluation of methyl aminolaevulinate-based photodynamic therapy in the management of actinic keratosis and basal cell carcinoma. Br J Dermatol. 2006;155(4):784–790.

