Abstract
Background
Tildrakizumab is an anti–interleukin-23 p19 monoclonal antibody approved for the treatment of adults with moderate-to-severe plaque psoriasis. Little real-world evidence is available regarding the effects of tildrakizumab on patients’ health-related quality of life (HRQoL) and patient-reported symptoms.
Objective
This real-world study of tildrakizumab evaluated changes in HRQoL and clinical symptoms in patients with psoriasis.
Materials and methods
In this Week (W)28 interim analysis of a 64-week Phase 4 study (NCT03718299), patients received tildrakizumab 100 mg at W0, W4, and every 12 weeks thereafter. Endpoints were improvement from baseline in Psychological General Well-Being Index (PGWBI), Dermatology Life Quality Index (DLQI), and Itch-, Pain-, and Scaling-Numerical Rating Scale scores through W28.
Results
Of 55 patients enrolled, 53 were assessed at W28. Mean (standard deviation [SD]) total PGWBI score improved from baseline to W28 (change, 3.7 [12.4]; p = .033), as did the positive well-being (1.0 [2.9]; p = .018) and general health (1.5 [2.2]; p < .001) domain scores. Mean (SD) DLQI score improved by −3.9 (4.3) at W4 and by −7.6 (5.1) at W28 (p < .001). Patient-reported symptoms improved starting at W4 (p < .001).
Conclusion
Tildrakizumab treatment improved HRQoL and patient-reported symptoms in patients with psoriasis in a real-world setting. Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT03718299
Introduction
Psoriasis is a chronic, multisystem disease that manifests most noticeably as inflammation of the skin (Citation1). The most common form, plaque psoriasis, is characterized by erythematous plaques with white-silver desquamation, which often appear on the skin of the knees, elbows, buttocks, trunk, and scalp (Citation1). Itch, pain, and scaling are common disease-related symptoms (Citation2,Citation3). Psoriasis and its symptoms, itching in particular, have substantial negative effects on patients’ health-related quality of life (HRQoL) (Citation2,Citation4–7). Patients with psoriasis report negative effects on sleep and rest, limitations in their daily activities including effects on mobility, and stigmatization and disruptions to their social interactions. Psoriasis can also have deleterious effects on mental health. Many patients with psoriasis experience depression, anxiety, or addiction (Citation5,Citation7).
Tildrakizumab is an anti–interleukin-23 p19 monoclonal antibody approved in the United States for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy (Citation8,Citation9). In 2 large Phase 3, multicenter, randomized clinical trials (reSURFACE 1 [NCT01722331] and reSURFACE 2 [NCT01729754]), patients with moderate-to-severe plaque psoriasis treated with tildrakizumab had improvement from baseline at Week 12 in both psoriasis disease activity, measured using the Psoriasis Area and Severity Index (PASI), and in HRQoL, measured using the Dermatology Life Quality Index (DLQI), compared with patients receiving placebo (Citation8). Post hoc analyses of the relationship between improvement in PASI and DLQI scores in reSURFACE 1 and reSURFACE 2 showed that patients who achieved higher PASI response thresholds at Week 28 were more likely to achieve favorable DLQI responses relative to patients with lower Week 28 PASI responses, consistent with the notion that patients with greater disease activity may have had greater impairment of their HRQoL (Citation8,Citation10,Citation11).
In general, the relationship between clinical treatment of psoriasis and patients’ HRQoL is poorly understood and may be underestimated by clinicians (Citation12). There is therefore an unmet need for a greater understanding of this relationship to inform the choice of therapeutic intervention. However, little published real-world evidence has been available regarding HRQoL or general well-being in patients with moderate-to-severe plaque psoriasis treated with tildrakizumab. To address this, a Phase 4 study was undertaken to evaluate changes in HRQoL and severity of clinical symptoms in patients with psoriasis treated with tildrakizumab under real-world conditions. Results from the interim analysis after 28 weeks of treatment are described here.
Material and methods
Study design and patients
A Phase 4, multicenter, 64-week, uncontrolled, open-label, real-world study (NCT03718299) was performed to evaluate effects on HRQoL in patients with psoriasis treated with tildrakizumab. Eligible patients included immunocompetent patients aged ≥18 years with moderate-to-severe plaque psoriasis affecting ≥3% of total body surface area and diagnosed at least 6 months prior to study entry, who were candidates for phototherapy or systemic therapy. Patients with erythrodermic psoriasis or those with only pustular, guttate, or inverse psoriasis were excluded from the study. Full inclusion and exclusion criteria are provided in Table SI. All patients received tildrakizumab 100 mg at Week 0, Week 4, and every 12 weeks thereafter through Week 52. Assessments through the Week 28 interim analysis are reported here.
The study protocol and all amendments were approved by a central institutional review board. The study was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent before beginning the study.
Outcome assessments
Patients’ HRQoL was evaluated at baseline and at Weeks 4, 8, 12, 16, and 28 using the Psychological General Well-Being Index (PGWBI) and the DLQI. The PGWBI is designed to measure psychological well-being in the general medical population. This HRQoL instrument includes 22 questions representing 6 domains: anxiety, depressed mood, positive well-being, self-control, general health, and vitality. Responses are scored on a Likert scale, in which the answers range from ‘strongly agree’ to ‘strongly disagree’ with gradations in between. The total score ranges from 0 to 110; a higher score indicates greater psychological well-being. The DLQI, which measures HRQoL in patients with skin disease, contains 10 questions which assess patients’ perception of items including symptoms, feelings, daily activities, leisure activities, work or school, personal relationships, and treatment. Each question is scored on a 4-point Likert scale (0 = not at all/not relevant; 1 = a little; 2 = a lot; 3 = very much). Scores of individual items (0–3) are combined to yield a total score of 0–30; higher scores indicate greater impairment of a patient’s skin-related HRQoL (Citation13). Patient-reported severity of itch, pain, and scaling was measured using numerical rating scales (NRSs). The individual NRS instruments for itch, pain, and scaling are all simple, self-administered 11-point scales; scores range from 0 to 10, and higher scores indicate worse symptoms (0 = no itching/pain/scaling to 10 = worst itching/pain/scaling imaginable).
The primary efficacy endpoint in this study was change in HRQoL as measured by the change from baseline in PGWBI at Week 28. Secondary efficacy endpoints included change from baseline in PGWBI score over time (Weeks 4, 8, 12, and 16); change from baseline in DLQI and Itch-, Pain-, and Scaling-NRS scores over time; and proportions of patients with a DLQI score of 0 or 1 (representing no negative effect of psoriasis on patients’ quality of life), DLQI ≤5, and ≥5-point reduction in DLQI from baseline over time.
Safety through Week 28 was assessed from treatment-emergent adverse events (TEAEs) at Weeks 4, 8, 12, 16, and 28.
Statistical analysis
Screening of 60 patients was considered sufficient, and no formal sample size calculations were performed. Results of HRQoL assessments were analyzed for the intention-to-treat (ITT) population, which consisted of all patients who were enrolled in the study and assigned to receive tildrakizumab. The PGWBI and DLQI results are reported as observed; missing data were not imputed. Differences between postbaseline and baseline values were analyzed using paired Student’s t-tests. Descriptive statistics were calculated for the absolute values and percentage changes from baseline in Itch-, Pain-, and Scaling-NRS scores. The safety population, which consisted of all randomized patients who received at least 1 dose of study treatment, was used for safety analyses.
Results
Patients
Of the 60 patients screened, a total of 55 patients were enrolled in this study. Of these, 28 (50.9%) were male and 52 (94.5%) were White; the mean age was 48.6 years (). Of the 55 patients enrolled, 53 (96.4%) were assessed for PGWBI, DLQI, and Itch-, Pain-, and Scaling-NRS scores at Week 28. Demographics and baseline characteristics of the ITT population are summarized in .
Table 1. Demographics and baseline characteristics of the ITT population.
Health-related quality of life assessments
Psychological General Well-Being Index
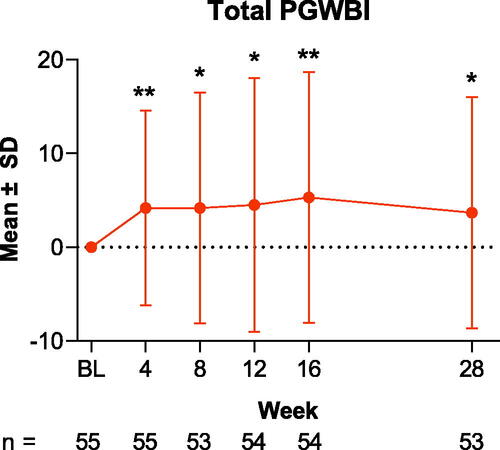
The primary endpoint of change from baseline in PGWBI total score at Week 28 was met; the mean (SD) total score increased from 78.1 (14.1) at baseline to 82.2 (12.9) at Week 28, a mean absolute change of 3.7 (SD, 12.4; mean percentage change, 7.3%; p = .033; ). Improvement was evident as early as Week 4, when total PGWBI score was 82.3 (13.1); the mean absolute change at Week 4 was 4.2 (10.4), and the mean percentage change was 7.2% (p = .004).
Figure 1. Change from baseline in total PGWBI scores through Week 28 in patients treated with tildrakizumab. Data are shown as the mean for the intention-to-treat population; error bars represent the standard deviation. *p < .05; **p < .01. BL: baseline; PGWBI: Psychological General Well-Being Index; SD: standard deviation.
Among the individual PGWBI domain scores, patients achieved improvement from baseline in the positive well-being domain score (mean absolute change, 1.0 [2.9]; mean percentage change, 13.0%; p = .018; ). The general health domain score also improved from baseline to Week 28; the mean absolute change was 1.5 (2.2), and the mean percentage change was 20.0% (p < .001; ).
Figure 2. Change from baseline in PGWBI domain scores through Week 28 in patients treated with tildrakizumab. Results are shown for PGWBI domains (A) positive well-being, (B) general health, (C) anxiety, and (D) vitality. Data are presented as the mean for the intention-to-treat population; error bars represent the standard deviation. *p < .05; **p < .01; ***p < .001. BL: baseline; PGWBI: Psychological General Well-Being Index; SD: standard deviation.
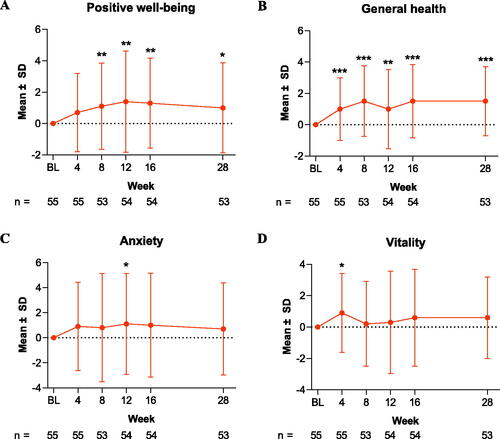
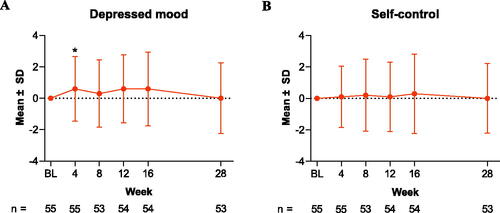
For the anxiety and vitality domain scores, the changes from baseline observed were not statistically significant. At Week 28, the mean absolute/percentage change was 0.7 (3.7)/8.6% (p = .174) for the anxiety domain and 0.6 (2.6)/7.9% (p = .097) for the vitality domain (). The depressed mood and self-control domain scores did not change from their baseline values at Week 28; the mean absolute change was 0.0 (2.3) and 0.0 (2.2) for depressed mood and self-control, respectively (). Sensitivity analyses using LOCF imputation yielded similar results (Table SII).
Figure 3. Change from baseline in (A) PGWBI depressed mood and (B) self-control scores through Week 28 in patients treated with tildrakizumab. Data are shown as the mean for the intention-to-treat population for PGWBI domains (A) depressed mood and (B) self-control. Error bars represent the standard deviation. *p < .05. BL: baseline; PGWBI: Psychological General Well-Being Index; SD: standard deviation.
Dermatology Life Quality Index
As shown in , the mean (SD) DLQI score decreased from 9.4 (5.2) at baseline to 5.5 (4.3) at Week 4; the mean absolute change was −3.9 (4.3) and the mean percentage change was −37.1% (p < .001). At Week 28, the mean DLQI score was reduced to 1.7 (1.9); the mean absolute change was −7.6 (5.1), and the mean percentage change was −76.5% (p < .001). The proportion of patients achieving a DLQI score of 0 or 1 (no negative effect of psoriasis on patients’ quality of life) at Week 28 was 52.8% (). The proportion of patients with a DLQI score ≤5 and the proportion of patients with a ≥5-point reduction in DLQI score from baseline at Week 28 was 94.3% (p < .001) and 81.4% (p < .001), respectively (). Sensitivity analyses using LOCF imputation yielded similar results (Table SII).
Figure 4. DLQI scores in patients treated with tildrakizumab. (A) Mean DLQI scores through Week 28 of treatment are presented. (B) Proportions of patients (n/N [%]) with a DLQI score of 0/1, a DLQI score of ≤5, or a ≥5-point reduction in DLQI score from baseline at Week 28 are shown. Panel A, *p < .001. Data are from the intention-to-treat population. Per (A), statistical significance was calculated per change from baseline. BL: baseline; DLQI: Dermatology Life Quality Index; SD: standard deviation.
![Figure 4. DLQI scores in patients treated with tildrakizumab. (A) Mean DLQI scores through Week 28 of treatment are presented. (B) Proportions of patients (n/N [%]) with a DLQI score of 0/1, a DLQI score of ≤5, or a ≥5-point reduction in DLQI score from baseline at Week 28 are shown. Panel A, *p < .001. Data are from the intention-to-treat population. Per (A), statistical significance was calculated per change from baseline. BL: baseline; DLQI: Dermatology Life Quality Index; SD: standard deviation.](/cms/asset/d6d893e7-d6ba-464d-a90c-9f50080021c2/ijdt_a_2200872_f0004_c.jpg)
Patient-reported symptoms
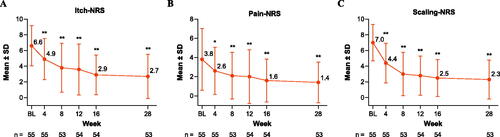
Patient-reported itch, pain, and scaling improved from baseline through Week 28. As shown in , the mean ± SD Itch-NRS score improved from 6.6 ± 2.6 at baseline to 4.9 ± 2.6 at Week 4 (−19.8%) and to 2.7 ± 2.8 at Week 28 (−57.4%; both p < .001). The Pain-NRS score (mean ± SD) was 3.8 ± 3.2 at baseline and decreased to 2.6 ± 2.5 by Week 4 (−10.0%; p = .001) and to 1.4 ± 2.1 by Week 28 (−44.8%; p < .001; ). The mean ± SD Scaling-NRS score of 7.0 ± 2.3 at baseline improved to 4.4 ± 2.5 at Week 4 (−36.7%) and to 2.3 ± 2.5 at Week 28 (−66.8%; both p < .001; ). Sensitivity analyses using LOCF imputation yielded similar results (Table SIII).
Figure 5. NRS scores in patients treated with tildrakizumab. Mean ± SD (A) Itch-NRS, (B) Pain-NRS, and (C) Scaling-NRS scores through Week 28. Data are from the intention-to-treat population. Missing data were not imputed; error bars represent the SD. *p < .05, **p < .001. Statistically significant change from baseline based on Student’s t-test. BL: baseline; NRS: Numerical Rating Scale; SD: standard deviation.
Safety
TEAEs were reported in 31 (56.4%) patients. Serious TEAEs were infrequent, occurring in 3 (5.5%) patients. No TEAEs were considered to be related to tildrakizumab. The most frequently reported TEAEs were skin and subcutaneous tissue disorders (20%), infections and infestations (14.5%), musculoskeletal and connective tissue disorders (10.9%), and gastrointestinal disorders (10.9%). Treatment was discontinued for 1 patient (1.8%) due to a TEAE.
Discussion
In this interim analysis, treatment with tildrakizumab in a real-world setting resulted in improved HRQoL in patients with moderate-to-severe psoriasis. Patients experienced improvements from baseline in DLQI and total PGWBI scores as early as Week 4, and these improvements were maintained through Week 28.
The PGWBI scores for the general health and positive well-being domains improved by Week 4 and Week 8, respectively. However, no statistically significant improvement from baseline to Week 28 was observed for the PGWBI domains for anxiety, vitality, depressed mood, and self-control. Tildrakizumab treatment improved patient-reported itching, pain, and scaling beginning as early as Week 4, and improvement was maintained through Week 28.
Patients with psoriasis have reported decreased HRQoL and often experience clinically relevant stress, anxiety, and depression (Citation5). Clinical trials have often focused on the effects of skin clearance on the HRQoL of patients with psoriasis. Complete skin clearance has been associated with higher scores on measures of HRQoL compared with almost complete clearance (Citation14,Citation15). Data pooled from 3 multinational Phase 3 trials of risankizumab in patients with moderate-to-severe psoriasis (UltIMMa-1, UltIMMa-2, and IMMvent) showed that patients who achieved high levels of skin clearance had greater improvements in HRQoL as assessed by DLQI and symptom control (Citation16). Post hoc analysis of 2 Phase 3, randomized, 52-week, double-blind clinical trials of secukinumab vs ustekinumab in patients with moderate-to-severe plaque psoriasis (CLEAR [NCT02074982] and SCULPTURE [NCT01406938]) revealed a correlation between PASI and DLQI (Citation17). A systematic review and meta-analysis of published articles relating to disease-modifying antirheumatic drugs including biologics (infliximab, secukinumab, and ustekinumab) used in the treatment of psoriasis showed that improvement in HRQoL as assessed by DLQI was associated with a higher PASI response in patients with moderate-to-severe psoriasis (Citation18). Additionally, the impact of the efficacy of tildrakizumab treatment on quality of life in patients with moderate-to-severe plaque psoriasis over 28 weeks was evaluated in 2 separate post hoc analyses of the 2 large Phase 3, multinational, randomized clinical trials of tildrakizumab (reSURFACE 1 [NCT01722331] and reSURFACE 2 [NCT01729754]) (Citation10,Citation19). The absolute PASI scores correlated with the DLQI total scores (Citation19), and higher PASI response levels were associated with more favorable DLQI scores (0/1) compared with lower PASI responses (Citation10).
Controlled clinical trials, which apply strict inclusion and exclusion criteria for enrolling patients, may not fully represent or characterize the patient population found in real-world clinical practice (Citation20). Real-world data may provide additional insight. Clinical and HRQoL data collected through the Psocare project in Italy showed that the PGWBI and DLQI indexes correlated with the European Quality of Life Five Dimension (EQ-5D), a health status index (Citation21). Post hoc analyses from the multinational observational PSOriasis Treated with BIOlogics in REAL Life (PSO-BIO-REAL) study showed that the HRQoL of patients with moderate-to-severe plaque psoriasis improved with skin clearance; however, complete correlation between PASI response and patient-reported outcomes was not established (Citation14). Further, this analysis did not include data on the newer biologics brodalumab, ixekizumab, guselkumab, tildrakizumab, and risankizumab (Citation14). To date, there has been little published real-world evidence regarding HRQoL in patients with moderate-to-severe plaque psoriasis treated with tildrakizumab.
Among psoriasis-related symptoms, itch is reported to be the greatest contributor to reductions in patients’ emotional well-being, daily activity, and sleep (Citation6). A retrospective database analysis conducted using both physician and patient-reported data from the Adelphi Real World 2011 and 2013 Psoriasis Disease Specific Programmes found that increased severity of itch, pain, and scaling was associated with both increased disease severity and reduced HRQoL and work productivity, supporting the importance of including key clinical symptoms of psoriasis as patient-reported outcomes in psoriasis clinical trials (Citation2). Results from clinical trials with biologics (brodalumab, ixekizumab, risankizumab, secukinumab, and guselkumab) showed that the improvement in psoriasis symptoms is an important contributor to the achievement of successful outcomes in patients with moderate-to-severe psoriasis; however, real-world data are scarce (Citation22–27). Interestingly, in the recently published real-life prospective ProLOGUE study of brodalumab, persistent itching prevented HRQoL improvement in Japanese patients with psoriasis despite the achievement of clear or almost clear skin, confirming the evidence from the clinical trials (Citation28).
A limitation of this analysis is the relatively small size of the patient population. Additionally, it is not clear from the data whether tildrakizumab treatment may affect patients’ HRQoL over a longer time horizon. Improvement in the DLQI, PGWBI, or any of the associated domain scores should continue to be evaluated with further follow-up.
In conclusion, for patients with moderate-to-severe plaque psoriasis in real-world clinical practice, tildrakizumab treatment resulted in statistically significant improvements from baseline in measures of HRQoL. Improvements in DLQI, total PGWBI, and patient-reported symptom severity scores were noted as early as Week 4 of treatment and were maintained through Week 28. Additional research is recommended to further guide clinicians in choosing optimal treatment strategies to improve HRQoL in patients with moderate-to-severe psoriasis.
Supplemental Material
Download PDF (133.5 KB)Acknowledgements
We thank Drs. Tina Bhutani, John Koo, and Stephen J Rozzo for contributions to the studies. Portions of these data were previously presented at the American Academy of Dermatology 2022 Annual Meeting (AAD 2022) in Boston, MA, on March 25–29, 2022, and at the 2022 Winter Clinical Dermatology Conference in Koloa, HI, on January 14–19, 2022.
Disclosure statement
NB is an adviser, consultant, and investigator for AbbVie, Almirall, Arcutis, Beiersdorf, Biofrontera, Bristol Myers Squibb, Boehringer Ingelheim, Cara, Dermavant, EPI Health, Ferndale, Galderma, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, LEO Pharma, Lilly, Ortho, Pfizer, Regeneron, Sanofi, Sun Pharma, and Verrica. JH has been a speaker, adviser, and consultant for AbbVie, Amgen, Celgene, Lilly, Janssen, and Novartis; an adviser for Galderma, Mayne, and Sanofi Regeneron; an adviser and consultant for Ortho Dermatologic; and a speaker and adviser for Sun Pharma. BS is an employee of Sun Pharmaceutical Industries, Inc. JGV reports nothing to disclose.
Data availability statement
Data and other documents will be made available after publication, with no end date, to anyone who submits a reasonable request to the study sponsor.
Additional information
Funding
References
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):1–850.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21(10):13030/qt1x16v3dg.
- de Korte J, Sprangers MA, Mombers FM, et al. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9(2):140–7.
- Bhutani T, Patel T, Koo B, et al. A prospective, interventional assessment of psoriasis quality of life using a nonskin-specific validated instrument that allows comparison with other major medical conditions. J Am Acad Dermatol. 2013;69(2):e79–e88.
- Nowowiejska J, Baran A, Grabowska P, et al. Assessment of life quality, stress and physical activity among patients with psoriasis. Dermatol Ther. 2022;12(2):395–406.
- Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62.
- Zhong H, Yang H, Mao Z, et al. Impact of moderate-to-severe psoriasis on quality of life in China: a qualitative study. Health Qual Life Outcomes. 2021;19(1):271.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288.
- ILUMYA® (tildrakizumab-asmn). [full prescribing information]. Cranbury (NJ): Sun Pharmaceutical Industries, Inc.
- Blauvelt A, Sofen H, Papp K, et al. Tildrakizumab efficacy and impact on quality of life up to 52 weeks in patients with moderate-to-severe psoriasis: a pooled analysis of two randomized controlled trials. J Eur Acad Dermatol Venereol. 2019;33(12):2305–2312.
- Strober B, Greenberg JD, Karki C, et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the corrona psoriasis registry. BMJ Open. 2019;9(4):e027535.
- Meneguin S, de Godoy NA, Pollo CF, et al. Quality of life of patients living with psoriasis: a qualitative study. BMC Dermatol. 2020;20(1):22.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994 May;19(3):210–216.
- Lacour JP, Bewley A, Hammond E, et al. Association between patient- and physician-reported outcomes in patients with moderate-to-severe plaque psoriasis treated with biologics in real life (PSO-BIO-REAL). Dermatol Ther. 2020;10(5):1099–1109.
- Viswanathan HN, Chau D, Milmont CE, et al. Total skin clearance results in improvements in health-related quality of life and reduced symptom severity among patients with moderate to severe psoriasis. J Dermatolog Treat. 2015;26(3):235–239.
- Ryan C, Puig L, Zema C, et al. The impact of achieving and maintaining near-complete or complete skin clearance on patient quality of life in moderate-to-severe psoriasis. J Am Acad Dermatol. 2023;88(1):169–172.
- Gerdes S, Korber A, Biermann M, et al. Absolute and relative psoriasis area and severity index (PASI) treatment goals and their association with health-related quality of life. J Dermatolog Treat. 2020;31(5):470–475.
- Puig L, Thom H, Mollon P, et al. Clear or almost clear skin improves the quality of life in patients with moderate-to-severe psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2017;31(2):213–220.
- Gordon KB, Reich K, Crowley JJ, et al. Disease activity and treatment efficacy using patient-level psoriasis area and severity index scores from tildrakizumab phase 3 clinical trials. J Dermatolog Treat. 2022;33(1):219–228.
- Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774.
- Spandonaro F, Altomare G, Berardesca E, et al. Health-related quality of life in psoriasis: an analysis of psocare project patients. G Ital Dermatol Venereol. 2011;146(3):169–177.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52.
- Augustin M, Lambert J, Zema C, et al. Effect of risankizumab on patient-reported outcomes in moderate to severe psoriasis: the UltIMMa-1 and UltIMMa-2 randomized clinical trials. JAMA Dermatol. 2020;156(12):1344–1353.
- Miyagi T, Kanai Y, Murotani K, et al. Itch as a critical factor in impaired health-related quality of life in patients with plaque psoriasis achieving clear or almost-clear skin: analysis of the single-arm, open-label, multicenter, prospective ProLOGUE study. JAAD Int. 2022;8:146–153.

