Dear Editor,
Vitiligo is a depigmentation disorder resulting from destruction of melanocytes. The pathogenesis of vitiligo is widely considered to be a type 1 skewed immune response that CD8+ T cells and their release of interferon (IFN)-γ are important mediators (Citation1). Atopic dermatitis (AD) is predominantly characterized by a type 2 immune response with also important role of T helper (Th) 22, Th17 and Th1 cytokines in different subtypes. Recent epidemiological analysis shows an increased risk of vitiligo in AD patients (Citation2), disclosing the potential association between vitiligo and AD. Silverberg et al. (Citation3) speculated that the pro-inflammatory state in AD might predispose toward melanocyte destruction and constant scratching of AD lesions could induce a Koebner effect in vitiligo. Consistent with this speculation, Martins et al. (Citation4) revealed a more complex immune network in vitiligo with an increased expression of Th2 response except for Th1, and their combination can potentiate the recruitment of CD8+ T cells into the skin. Furthermore, thymic stromal lymphopoietin (TSLP) and interleukin (IL)-4 gene susceptibilities (Citation5–7) and increased serum IL-33 (Citation8) have been identified in vitiligo, all of which linked with a Th2 immune response. These results indicate that vitiligo shares an overlapping genetic and immune mechanisms with AD, thus leading to their concurrent.
Upadacitinib is an oral JAK inhibitor engineered to have increased selectivity for JAK1 vs other JAKs. By selectively inhibiting JAK1, upadacitinib abrogates the signaling of many proinflammatory mediators, including IL-4, IL-13, IL-22, IL-31, and TSLP, thus contributing to skin clearance and itch relief of AD (Citation9). In vitiligo, JAK1 has been confirmed to be significantly elevated in the lesions and decreased after UVB treatment (Citation10). JAK1 inhibitors could block the pathway of IFN-γ and restore the epidermal homeostasis, then reducing melanocyte destruction and pigment loss (Citation4). Theoretically, JAK1 inhibitors could have dual effects on patients concurrent with AD and vitiligo.
In this article, we report a 16-year-old boy who was diagnosed with AD since childhood, presented with rapidly progressive vitiligo during last four months. Physical examination revealed multiple white patches with irregular shapes and blurred margins distributed on his face, neck, chest and extremities (Vitiligo Disease Activity score [VIDA] = 2; Vitiligo Area Scoring Index [VASI] = 0.35). Additionally, he exhibited dry skin with erythema, papules and slight scales on the neck and extremities (Eczema Area and Severity Index [EASI] = 10.5; Number Rating Scale of Pruritus [NRSp] = 6). The patient suffers from allergic rhinitis, while his family history is unremarkable. Laboratory tests suggested the elevation of immunoglobulin E (IgE). Tests for trace elements, thyroid function and auto-antibodies were all normal.
After exclusion of contraindications (viral hepatitis, tuberculosis, tumor, and severe infection) and full informed consent, treatment began with upadacitinib 15 mg daily, combined with crisaborole on both white patches and dermatitis, and meanwhile he was instructed to reinforce skin care. After one month of treatment, no new white patches appeared and there was repigmentation on his face and neck (). After four months, there were nearly 90% repigmentation of his face and neck (), 60% repigmentation of the chest, and only a little repigmentation of the extremities. Pruritus of AD disappeared within 24 h after treatment (NRSp = 0) and lesions improved significantly after four months (EASI = 4.1). During the treatment, regular examination of blood routine, blood biochemistry and coagulation showed no abnormalities, while his acne worsened which relieved with topical medication. The dose of upadacitinib was halved after four months of induction treatment. No recurrence has occurred in the next three months and the patient is still under follow-up.
Figure 1. Clinical pictures of rash during treatment. (a) Facial white patch at baseline, with irregular shapes and blurred margins. (b) One month after upadacitinib, the size of the white patch was reduced and repigmentation appeared on the edge. (c) Three months after upadacitinib, further reduction in the size and more repigmentation. His acne worsened at the same time. (d) Four months after upadacitinib, there was nearly 90% repigmentation of his facial white patch. (e) Cervical white patch at baseline. (f) one month after upadacitinib, the color of the white patch reduced. (g)&(h) Three and four months after upadacitinib, the rash showed complete repigmentation.
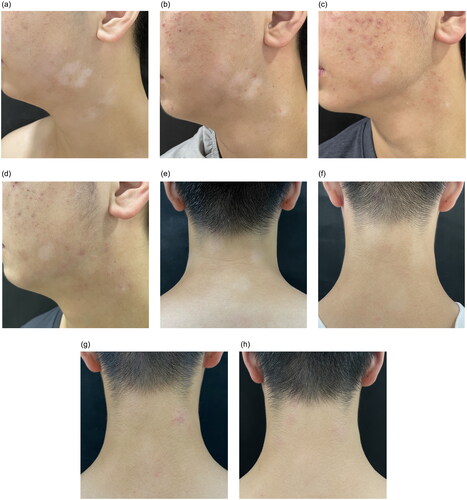
The present patient concurrent rapidly progressive vitiligo with AD. Treatment with upadacitinib resulted in expected relief of AD. Meanwhile, his vitiligo stopped progressing after treatment and gradually re-pigmented. Notably, the curative effect was better on exposed areas than the unexposed, which corresponded with the responses of other JAK inhibitors. Lucy et al (Citation11) propose that repigmentation in vitiligo requires both immune suppression (achieved with JAK inhibitors) and melanocyte stimulation (via low-dose nbUVB or sunlight). Therefore, combined phototherapy can be tried in this patient, especially on the trunk and extremities, for further repigmentation.
In conclusion, JAK1 inhibitor upadacitinib shows excellent effects on both AD and vitiligo, thus can be a preferred treatment option when two diseases co-exist.
Informed consent
The patient in this manuscript has given written informed consent to publication of his case details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not application to this article as no new data were created or analyzed in this study. Further details on the case can be available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Kim SR, Heaton H, Liu LY, et al. Rapid repigmentation of vitiligo using tofacitinib plus Low-Dose, narrowband UV-B phototherapy. JAMA Dermatol. 2018;154(3):1–3.
- Acharya P, Mathur M. Association of atopic dermatitis with vitiligo: a systematic review and meta-analysis. J Cosmet Dermatol. 2020;19(8):2016–2020.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35(3):283–289.
- Martins C, Migayron L, Drullion C, et al. Vitiligo skin T cells are prone to produce type 1 and type 2 cytokines to induce melanocyte dysfunction and epidermal inflammatory response through jak signaling. J Invest Dermatol. 2022;142(4):1194.e7–1205.e7.
- Imran M, Laddha NC, Dwivedi M, et al. Interleukin-4 genetic variants correlate with its transcript and protein levels in patients with vitiligo. Br J Dermatol. 2012;167(2):314–323.
- Cheong KA, Chae SC, Kim YS, et al. Association of thymic stromal lymphopoietin gene -847C>T polymorphism in generalized vitiligo. Exp Dermatol. 2009;18(12):1073–1075.
- Birlea SA, Jin Y, Bennett DC, et al. Comprehensive association analysis of candidate genes for generalized vitiligo supports XBP1, FOXP3, and TSLP. J Invest Dermatol. 2011;131(2):371–381.
- Vaccaro M, Cicero F, Mannucci C, et al. IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch Dermatol Res. 2016;308(7):527–530.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-Severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055.
- Nada HR, El Sharkawy DA, Elmasry MF, et al. Expression of janus kinase 1 in vitiligo & psoriasis before and after narrow band UVB: a case-control study. Arch Dermatol Res. 2018;310(1):39–46.
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77(4):675.e1–682.e1.

