Abstract
Background: Lichen planopilaris (LPP) is a lymphocytic primary cicatricial alopecia characterized by perifollicular erythema, follicular hyperkeratosis and scaring, resulting in permanent hair loss. Current treatment modalities, both topical and systemic, fail to achieve satisfactory and consistent results. As therapies fail to halt the inflammatory process, patients with LPP may face long-term disfigurement and significant psychological burden.
Purpose: To initiate an efficacious targeted therapy with good tolerability and low side effect profile that will allow hair regrowth and prevent the development to disfiguring alopecia.
Materials and methods: Here, we report on a case of rare LPP in a linear distribution (LLPP) involving the scalp and forehead failing to achieve satisfactory results with continued hair loss with multiple previous treatments.
Results: Complete hair regrowth was achieved 12 weeks after treatment with an anti-psoriatic, anti-interleukin (IL)-17A/F antibody (Taltz, Ixekizumab, Lilly). Patient continued to display sustained efficacy with no reported side effects until 12 months on treatment.
Conclusions: The present case underlines the viability of Ixekizumab as a possible first-line, targeted therapy for LPP and its variants with sustained efficacy. Multicenter trials are warranted to confirm the benefit of Ixekizumab as a successful targeted biologic treatment option for LPP and LLPP.
Lichen planopilaris (LPP) is a lymphocytic primary cicatricial alopecia characterized by perifollicular erythema, follicular hyperkeratosis and scaring, resulting in permanent hair loss (Citation1). Current treatment modalities, both topical and systemic, fail to achieve satisfactory and consistent results (Citation2–4). As therapies fail to halt the inflammatory process, patients with LPP may face long-term disfigurement and significant psychological burden (Citation5). Thus, efficacious, safe and targeted therapies are a high priority in preventing disfiguring alopecia in patients with LPP. Here, we report on a case of rare LPP in linear distribution (LLPP) involving the scalp and forehead achieving complete hair regrowth after 12 weeks of treatment with an anti-psoriatic, anti-interleukin (IL)-17A/F antibody (Taltz, Ixekizumab, Lilly). The present case underlines the viability of Ixekizumab as a possible first-line, targeted therapy for LPP and its variants with sustained efficacy.
Case synopsis
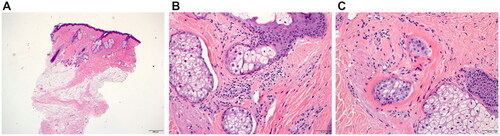
A 30-year-old woman of Iraqi origin presented with complaints of hair loss in the frontal area of her scalp and forehead of three months duration. The patches of hair loss were completely asymptomatic, non-pruritic, non-tender, and patient’s medical history was otherwise unremarkable. Treatments performed in private clinical practice included intralesional triamcinolone injections (1 session), plasma rich-platelet (PRP) and mesotherapy injections (2 sessions and 1 session, respectively), as well as oral spironolactone (50 mg daily) and topical minoxidil 5% for 3 months, all without improvement. Dermatological examination revealed a linear distribution of hair loss over the center of the frontal region of the scalp with underlying erythema extending down the upper forehead (). Clinical findings were suspicious of a scarring alopecia. All routine laboratory findings were within normal range apart from an asymptomatic pyuria that was treated with outpatient regimen for urinary tract infections with oral nitrofurantoin (50 mg, every 6 hours for 5 days). A 4-mm skin punch biopsy was taken from the frontal scalp and sent for hematoxylin and eosin (H and E) staining. Biopsy findings found very few hair follicles and sebaceous glands with evidence of perifollicular fibrosis consistent with LPP (). There was superficial perifollicular chronic inflammation not seen in the deeper levels. Fibrous streamers were noted without significant superficial lichenoid inflammation (). Histopathological features were compatible with a primary cicatricial alopecia. The differential diagnosis included fibrosing alopecia in a pattern distribution or localized linear scleroderma. Histological and clinical correlation supported LPP in a linear distribution. Considering recent publications for LPP showing success with other anti-psoriatic biologic agents, tildrakizumab (IL-23 monoclonal antibody) and adalimumab (anti-TNFa antibody), subcutaneous injection with Ixekizumab was initiated (Citation6,Citation7).
Figure 1. Patches of cicatricial alopecia involving the frontal scalp showing (A) Pre-treatment linear patch of hair loss with underlying erythema on the frontal scalp region (B) Pre-treatment well-defined patches of hair loss extending to parietal scalp. (C) Well-defined patch of hair loss on parietal scalp with white patches on background of erythema with mild perifollicular scaling on periphery, single hairs and loss of follicular ostia. (D) Pre-treatment linear depression on forehead. Post-treatment scalp (E) and (F) at week 20 after 10 doses of subcutaneous Ixekizumab showing complete hair regrowth with no evident erythema.
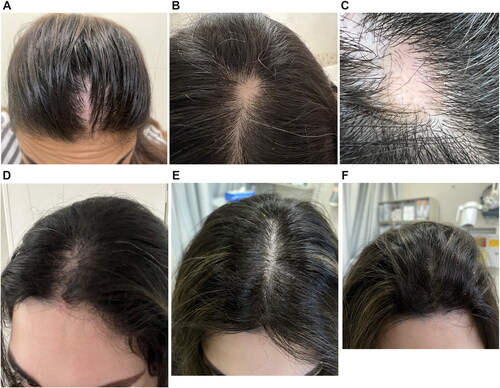
Figure 2. Histopathological examination from lesional scalp biopsy in (A) low power view of skin punch biopsy, examined vertically, showing mild follicular plugging and perifollicular fibrosis. There appears to be reduction in the hair density, with mild inflammation confined to the upper parts of the hair follicle. Some dilated eccrine ducts are also noted in the lower dermis (hematoxylin-eosin, original magnification ×4). (B) Mild perifollicular chronic inflammation with foci of interface inflammation (hematoxylin-eosin, original magnification ×20). (C) Focal area of perifollicular fibrosis (hematoxylin-eosin, original magnification ×20).
Ixekizumab is a humanized IgG4 monoclonal antibody that selectively binds IL-17A and inhibits its interaction with the IL-17A receptor (Citation8). Dosing of Ixekizumab was introduced as formally given for plaque psoriasis (160 mg s.c. then 80 mg s.c. every 2 weeks for 12 weeks then 80 mg s.c. every 4 weeks). This was combined with a class IV topical steroid (clobetasol dipropionate) alternating every two weeks with a topical steroid-sparing calcineurin inhibitor (tacrolimus monohydrate 0.1%), as previously used but unsuccessfully as monotherapy.
At follow-up examination (week 12; 8 doses), complete clinical improvement was noted using lichen planopilaris-activity index (IPPAI) of 0 (scale 1–10) in comparison to her initial visit with IPPAI 6 that was also confirmed using SALT score (2,4 × 2,4 cm used as 1% scalp surface) (Citation9) .The patient continued to display excellent response (IPPAI 0) with complete hair regrowth including improved appearance of the linear forehead depression at week 20 (10 doses) (). Consequently, patient stopped topical treatment and continued Ixekizumab monotherapy (80 mg s.c./month) without relapse or side effects as well as excellent tolerability.
Discussion
Controlling the inflammatory process in LPP and its subtypes is challenging and responses to current treatment modalities remain disappointing and cases often recalcitrant (Citation10,Citation11). LPP in a linear pattern (LLPP) has been described but is very rare especially when involving the scalp and forehead with poorly understood immunopathology (Citation12). However, histological features of LLPP are identical to classic LPP and its subtypes. Different treatment modalities have been described including topical or systemic immunosuppressants such as glucocorticosteroids, calcineurin inhibitors, cyclosporine A, azathioprine, methotrexate or hydroxychloroquine. These treatments are occasionally associated with significant, even life-threatening side effects due to lack of specific action as well as poor tolerability for some drugs (Citation13). Both LPP and psoriasis likely share several cytokine pathways (Th1, Th17), not including Th2. Thus, it is feasible to speculate that anti-psoriatic therapies may be effective in the treatment of lichen planus, LPP or LLPP.
While anti-psoriatic agents have been recently reported to yield results in LPP, treatment success of anti-IL17 treatment with Ixekizumab for LPP or LLPP have not been described in the literature as of yet (Citation6,Citation7). Here, we report the successful treatment of LLPP using Ixekizumab monotherapy effective within 12 weeks and showed a sustainable effect under treatment, for a total of 12 months, without any reported side effects. Despite the relentless nature of scarring alopecia’s, early deployment of therapy during the active phase of the disease, as seen in this case, is key to halting the inflammatory process and preventing long-term scarring and disfigurement.
In summary, we report the successful treatment of a severe case of adult LLPP early targeting IL-17A/F using Ixekizumab with rapid, sustainable efficacy, excellent safety and tolerability. Targeting IL-17A/F appears to be an effective, safe and well-tolerated biologic therapy to control and reverse the active phase of LLPP, an often recalcitrant, disfiguring cicatricial alopecia. Multicenter clinical trials or large cohort studies are warranted to confirm the benefit of Ixekizumab and other biologics as successful and targeted biologic treatment options for LPP and LLPP.
Consent form
The patient in this manuscript has given written informed consent to publication of their case details.
Author contributions
MS was involved in the conceptualization, clinical management and diagnosis of the patient as well as supervision and manuscript writing. HA drafted the original manuscript, subsequent review and editing as well as clinical management and follow up. MP reported the result of the histopathological evaluation.
Acknowledgements
Open Access funding provided by the Qatar National Library. Supported by MRC, Hamad Medical Corporation, Doha, Qatar.
Disclosure statement
MS has been a consultant, performed consultancy services or received research grants from Galderma, Novartis, Lilly, Pfizer, Merck, Sanofi-Aventis, Regeneron, Maruho, Toray, ZymoGenetics, L’Oreal, Leo, Avon, Baiersdorf. HA and MP do not declare any conflicts of interest.
Data availability statement
Data available upon request.
Additional information
Funding
References
- Bolduc C, Sperling LC, Shapiro J. Primary cicatricial alopecia: lymphocytic primary cicatricial alopecias, including chronic cutaneous lupus erythematosus, lichen planopilaris, frontal fibrosing alopecia, and Graham-Little syndrome. J Am Acad Dermatol. 2016;75(6):1–4.
- Ross EK, Tan E, Shapiro J. Update on primary cicatricial alopecias [published correction appears in J Am Acad Dermatol. 2005 Sep;53(3):496]. J Am Acad Dermatol. 2005;53(1):1–40.
- Tursen U, Api H, Kaya T, et al. Treatment of lichen planopilaris with mycophenolate mofetil. Dermatol Online J. 2004;10(1):24.
- Cevasco NC, Bergfeld WF, Remzi BK, et al. A case-series of 29 patients with lichen planopilaris: the Cleveland Clinic Foundation experience on evaluation, diagnosis, and treatment. J Am Acad Dermatol. 2007;57(1):47–53.
- Chiang YZ, Bundy C, Griffiths CE, et al. The role of beliefs: lessons from a pilot study on illness perception, psychological distress and quality of life in patients with primary cicatricial alopecia. Br J Dermatol. 2015;172(1):130–137.
- Trindade de Carvalho L, Meah N, Wall D, et al. Recalcitrant lichen planopilaris and frontal fibrosing alopecia responding to tildrakizumab. Dermatol Ther. 2020;33(4):e13694.
- Alam MS, LaBelle B. Treatment of lichen planopilaris with adalimumab in a patient with hidradenitis suppurativa and rheumatoid arthritis. JAAD Case Rep. 2020;6(3):219–221.
- Brembilla NC, Senra L, Boehncke WH. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front Immunol. 2018;9:1682.
- Chiang C, Sah D, Cho BK, et al. Hydroxychloroquine and lichen planopilaris: efficacy and introduction of Lichen Planopilaris Activity Index scoring system. J Am Acad Dermatol. 2010;62(3):387–392.
- Sperling LC, Nguyen JV. Commentary: treatment of lichen planopilaris: some progress, but a long way to go. J Am Acad Dermatol. 2010;62(3):398–401.
- Lyakhovitsky A, Amichai B, Sizopoulou C, et al. A case series of 46 patients with lichen planopilaris: demographics, clinical evaluation, and treatment experience. J Dermatolog Treat. 2015;26(3):275–279.
- Delacerda AB, Chandler RG, Wells MJ, et al. Isolated linear lichen planopilaris: extremely rare when limited to the scalp. Cutis. 2013;92(6):303–305.
- Kowal A, Warmińska J, Krasowska D. Immunosuppressive drugs in dermatology–benefits and threats. Ann Univ Mariae Curie Sklodowska Med. 2003;58(2):14–21.

