Abstract
Objective
Patients with atopic dermatitis (AD) have low treatment satisfaction. In this study, we evaluated the humanistic burden, treatment satisfaction, and treatment expectations in patients with AD in the United States.
Methods
Adults with AD recruited through the National Eczema Association and clinical sites completed a web-based survey comprising the Patient-Oriented SCORing Atopic Dermatitis (PO-SCORAD), Dermatology Life Quality Index; Work Productivity and Activity Impairment Questionnaire-Atopic Dermatitis; Treatment Satisfaction Questionnaire for Medication (TSQM); and answered questions on healthcare provider (HCP) visits, treatment history, and treatment goals. Descriptive analyses were performed to compare participants by severity.
Results
Among 186 participants (mean [standard deviation] age 39.7 [15.3] years, 79.6% female), 26.9%, 44.6%, and 26.3% of the participants had mild, moderate, or severe AD, respectively, based on PO-SCORAD. Greater disease severity was associated with a greater impact on work and daily life, decreased TSQM scores, and increased HCP visits. Corticosteroid topical cream or ointment (53.8%) and oral antihistamines (31.2%) were most commonly used for the treatment of AD. Participants reported declining/stopping/changing AD treatment due to the potential for side effects or lack of efficacy. ‘Leading normal lives’ (28.0%) and ‘being itch-free’ (33.9%) were important treatment goals.
Conclusions
Individuals with AD, especially severe disease, face a considerable humanistic burden even while using treatment.
Introduction
Atopic dermatitis (AD) is a chronic heterogeneous inflammatory skin disease (Citation1). In the United States (US), the prevalence of AD in adults is approximately 2–7% (Citation2–4), i.e., 6.6–23.2 million patients as calculated from 2020 US census data (Citation5). Among these patients, the proportion of moderate-to-severe AD is approximately 30% (Citation6). Moreover, among skin diseases, AD has the highest disease burden globally as measured by disability-adjusted life-years (Citation7).
AD poses detrimental effects on patients’ lives by impacting health and quality of life (QoL) as well as psychological, social, and occupational aspects (Citation8). A recent US population-based survey reported that adults with AD (vs. those without AD) were more likely to rate their overall health as ‘only fair’/’poor’, and satisfaction with life as ‘somewhat dissatisfied’/’very dissatisfied’ (Citation9). The prevalence of self-reported healthcare-diagnosed anxiety or depression is also higher in adults with AD vs. those without AD (40.0% vs. 17.5%) (Citation10). Patients with AD in the US have also reported lower QoL and higher absenteeism, presenteeism, and overall work and activity impairment than matched non-AD controls (Citation11). AD also imposes a substantial financial burden (Citation8) as patients with AD incur significant out-of-pocket costs related to AD management (Citation12), and have higher mean direct ($24,401 vs. $14,619) (Citation13) and indirect costs ($8907 vs. $6517) (Citation11) than those without AD.
Among AD treatments, topical agents such as moisturizers, corticosteroids, calcineurin inhibitors, and phosphodiesterase-4 inhibitors are often baseline therapeutic options (Citation14), while phototherapy (Citation15) and systemic immunomodulatory agents such as cyclosporine, azathioprine, mycophenolate mofetil, methotrexate or systemic corticosteroids may be considered if topical treatments inadequately control AD, or if the patient’s QoL is substantially impacted (Citation15,Citation16). In the past five years, several treatments for AD have been approved by the Food and Drug Administration. These include biologics such as dupilumab and tralokinumab, oral small molecules such as upadacitinib and abrocitinib, and the topical small molecule ruxolitinib (Citation17). Additionally, several agents administered via the injectable, oral, or topical route for AD treatment are being investigated (Citation18).
Although treatment options are expanding, patients’ satisfaction with traditional topical and systemic treatment options is low, and studies exploring or discussing newer treatment options are limited (Citation19,Citation20). Data on the humanistic burden and expectation of patients with AD in the US are limited. Thus, this study aimed to evaluate the humanistic burden of AD, treatment satisfaction, and treatment expectations for patients with AD, both overall and stratified by disease severity.
Methods
Study design and participant recruitment
This is a cross-sectional, non-interventional, US-based, web-based survey of adult participants with AD, conducted from September 2020 to February 2021. The study received institutional review board (IRB) approval from Advarra IRB (Columbia, MD; Advarra study number: Pro00041638). The recruitment methodology has been published previously (Citation21). Participants were recruited through the National Eczema Association (NEA) advocacy group or via one of four clinical sites. Screening questions were used to determine participant eligibility. During the screening, participants were asked whether they had been offered or recommended systemic AD therapy in the past two years. Participants answering ‘no’ were approximated to have mild AD, and participants answering ‘yes’ were approximated to have moderate or severe AD. Enrollment was monitored to ensure that ≤25% of the study participants had mild AD (not offered a systemic AD therapy in the past two years). Systemic medications included oral or injectable corticosteroids, immunosuppressants, biologics (dupilumab), oral antihistamines, anti-microbial medications, and anti-viral medications. Once enrolled in the study, the severity of AD (mild, moderate, or severe) was clarified with use of Patient-Oriented SCORing Atopic Dermatitis (PO-SCORAD).
Eligible participants received a unique link to the web survey via e-mail and provided electronic consent. The survey comprised approximately 100–150 questions (depending on skip logic). Those who did not begin or complete the survey received at least one reminder e-mail. Respondents received $40 in the form of a gift card for completing the survey.
Inclusion and exclusion criteria
Participants were included if they were ≥18 years of age; lived in the US; had a diagnosis of AD for ≥12 months; could use a computer or smartphone and access the internet; provided consent; and could speak, read, and write English sufficiently to participate. Participants with a historic diagnosis of lupus erythematosus, psoriasis, and/or any form of skin cancer were excluded.
Measures
Data on demographics and clinical characteristics, employment status, and the impact of AD on employment were evaluated. Participants also completed the following patient-reported outcome measures: PO-SCORAD, Dermatology Life Quality Index (DLQI), Work Productivity and Activity Impairment Questionnaire-Atopic Dermatitis (WPAI-AD), and Treatment Satisfaction Questionnaire for Medication (TSQM).
PO-SCORAD considers the same items as the SCORAD (the extent and severity of AD lesions and the severity of itch and sleep disturbance; range, 0–103). Based on SCORAD index results, AD is classified into mild (≤27), moderate (≥28–≤56), and severe (≥57) (Citation22). DLQI is a 10-item questionnaire scored on a scale of 0–30, with higher scores representing greater impairment of the patient’s QoL (Citation23). WPAI-AD is expressed as percentage of impairment, with higher numbers indicating greater impairment and lesser productivity (Citation24). TSQM is a 14-item self‐reported questionnaire divided into four domains: effectiveness, side effects, convenience, and global satisfaction. Using the provided scoring equation, total scores in each domain are calculated from 0 to 100. A higher score indicates better satisfaction in the domain (Citation25). Participants also reported their healthcare provider (HCP) type, visit frequency, treatments used for AD, reasons for declining/discontinuing/changing AD treatment, and treatment goals, and expectations.
Statistical analysis
Descriptive analyses were performed on data for the overall sample and stratified by severity as per PO-SCORAD scores (≤27: mild AD, ≥28 to ≤56: moderate AD, and ≥57: severe AD) (Citation22). Mean and standard deviation (SD) were presented for continuous variables. Frequency and percent distribution by category were presented for categorical variables. To evaluate differences across AD severity groups, Chi-square tests were used for categorical data and t-tests and general linear models were used for continuous data. Analysis of variance with Scheffe’s test was used for multiple treatment comparisons. All statistical tests used a two-sided significance level of .05. Data were analyzed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC).
Results
Participant disposition
Overall, 511 individuals were invited to participate in the survey, and 389 completed the screening questions. Among the 389 individuals, 183 were ineligible and 20 started the survey but did not complete it. The most common reasons for exclusion were ‘did not endorse having a dermatologic condition’ (n = 53; 10.4%), ‘did not endorse having AD/eczema’ (n = 25; 4.9%), and ‘diagnosed with AD <12 months ago’ (n = 25; 4.9%). In total, 186 participants (recruitment: NEA, n = 111; clinical sites, n = 75) were included in the analysis.
Demographic characteristics
The mean (SD) PO-SCORAD score was 42.1 (20.5). Out of 186 participants, most had moderate AD (n = 83; 44.6%), followed by mild AD (n = 50; 26.9%), and severe AD (n = 49; 26.3%). Demographic characteristics of the overall sample and stratified by PO-SCORAD AD severity are detailed in . Overall, the mean (SD) age was 39.7 (15.3) years, and the majority were female (n = 148; 79.6%). Approximately, half of the participants were White (n = 99; 53.2%) and were single/never married (n = 92; 49.5%). Participants with mild AD were older than those with moderate or severe AD (mean [SD] 42.8 [16.5] vs. 40.6 [14.9] vs. 35.1 [13.5]; p = .0311). The greatest proportion of participants among those with mild or moderate AD was White (n = 35; 70.0% and n = 47; 56.6%), respectively. Asian participants made up the greatest proportion of those with severe AD (n = 22; 44.9%). Based on the image selected in the PO-SCORAD, most participants reported a light skin tone (n = 79; 42.5%), followed by medium (n = 68; 36.6%), and dark (n = 38; 20.4%) (missing, n = 1 [0.5%]).
Table 1. Participant demographic characteristics overall and by AD severity.
Quality of life, employment status, and impact of AD on employment
The mean (SD) DLQI score was 9.9 (7.4); scores increased as disease severity worsened (mild: 2.9 [3.6], moderate: 9.7 [5.7], severe: 17.7 [5.2]; p < .0001). A very large or extremely large effect on their lives was reported by only 8.0% of the patients with mild AD (n = 4) and almost all patients with severe AD (n = 47; 95.9%) ().
Table 2. Quality of life in patients with ADTable Footnotea.
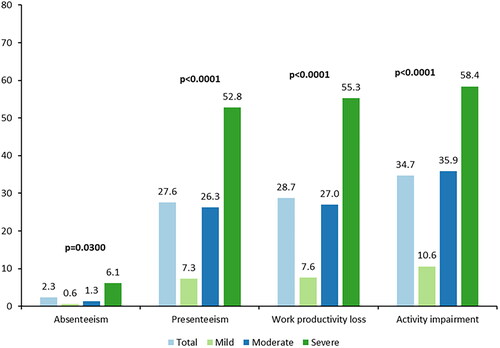
More than half of the participants (n = 100; 53.8%) were employed full-time and 16.7% (n = 31) were employed part-time (). Of those not employed (n = 47), 10.6% (n = 5) were not employed due to moderate or severe AD. Overall, 48.4% (n = 90) of the participants reported no effect on their career or work life due to AD. A higher proportion of participants with mild AD (n = 39; 78.0%) than those with moderate AD (n = 34; 41.0%) or severe AD (n = 15; 30.6%) reported no effect on career or work life (p < .0001). Significant differences between AD severity groups were also observed for statements regarding increased distraction at work, taking on a job with less seniority or responsibility after AD diagnosis, and earning less money than possible if the participant did not have AD (p < .05 for all) (). For employed participants, the mean (SD) scores for absenteeism, presenteeism, and work productivity loss were 2.3 (9.3), 27.6 (27.7), and 28.7 (28.4), respectively. The mean (SD) activity impairment score for all participants was 34.7 (28.9). Participants with severe AD had higher scores (p < .05) for all four WPAI domains than those with mild and moderate AD ().
Table 3. Work impact of ADTable Footnotea.
Figure 1. Work Productivity and Activity Impairment Questionnaire – Atopic Dermatitisa. Recall period: past seven days. aScores from the PO-SCORAD rounded to the first integer were used to define severity; scores ≤27 indicate mild AD, scores ≥28 to ≤56 indicate moderate AD, and scores ≥57 indicate severe AD. Four participants were missing severity level categorization due to at least one missing item on the PO-SCORAD. Employed: n = 126. For absenteeism, presenteeism, and work productivity loss scores: total, n = 126; mild, n = 33; moderate, n = 59; severe, n = 32. For activity impairment score: total, n = 186; mild, n = 50; moderate, n = 83; severe, n = 49. AD: atopic dermatitis; PO-SCORAD: Patient-Oriented SCORing Atopic Dermatitis.
Overall, 40.3% (n = 75) of the participants reported no effect of AD on any of their educational activities, relationships, family plans, or leisure activities. Here too, patients with mild AD (n = 31; 62.0%) fared better than those with moderate AD (n = 31; 37.3%) or severe AD (n = 11; 22.4%; p = .0002). Disease severity affected all other aspects of life significantly, except performing parenting duties (p < .05 for all) ().
Treatment satisfaction of topical and systemic medications
Overall, in participants receiving topical medications, the mean (SD) TSQM global satisfaction, effectiveness, side effects, and convenience scores were 64.0 (20.5), 58.1 (20.3), 64.8 (31.7), and 67.3 (15.2), respectively (). Significant differences were observed in all four TSQM domains on stratification by disease severity (global satisfaction: p = .0039, effectiveness: p < .0001, side effects: p = .0423, and convenience: p = .003). Pairwise comparisons showed significantly higher scores for participants with mild AD for global satisfaction (vs. severe AD; p < .01), effectiveness (vs. severe AD; p < .001), and convenience (vs. moderate AD; p < .01 and vs. severe AD; p < .05). Significantly higher effectiveness scores were reported by participants with moderate AD vs. severe AD (p < .01).
Figure 2. Treatment Satisfaction Questionnaire Medication (TSQM Version 2.0), topical and systemic medications, overall and by disease severitya,b,c. (A, B) TSQM scores for topical and systemic medications, respectively. (A) Pairwise comparisons: global satisfaction – mild vs. severe (p < .01), effectiveness – mild vs. severe (p < .001) and moderate vs. severe (p < .01), convenience – mild vs. moderate (p < .01), and mild vs. severe (p < .05). (B) Pairwise comparisons: global satisfaction – mild vs. severe (p < .01), effectiveness – mild vs. moderate (p < .01), and mild vs. severe (p < .001). aTopical medications included: corticosteroid topical cream or ointment, topical calcineurin inhibitor, topical phosphodiesterase 4 inhibitor, antihistamine by topical cream or ointment, emollient or moisturizer, itch cream, gel or ointment, and cleansers. bSystemic medications included: corticosteroid by mouth or injection, immunosuppressant, biologics (dupilumab), antihistamine by mouth, anti-microbial medication, and anti-viral medication. cScores from the PO-SCORAD were used to define severity; scores ≤27 indicate mild AD, scores ≥28 to ≤56 indicate moderate AD, and scores ≥57 indicate severe AD.
Three and two participants for topical and systemic medications respectively are missing severity level categorization due to at least one missing item on the PO-SCORAD. Missing values were not included in %.In topical medications, for global satisfaction, effectiveness score, and convenience score: total, n = 171; mild, n = 44; moderate, n = 79; severe, n = 45. For side effects score: total, n = 40; mild, n = 5; moderate, n = 19; severe, n = 16. In systemic medications, for global satisfaction, effectiveness score, and convenience score: total, n = 113; mild, n = 20; moderate, n = 55; severe, n = 36. For side effects score: total, n = 40; mild, n = 5; moderate, n = 21; severe, n = 14. AD: atopic dermatitis; PO-SCORAD: Patient-Oriented SCORing Atopic Dermatitis; TSQM: Treatment Satisfaction Questionnaire Medication.
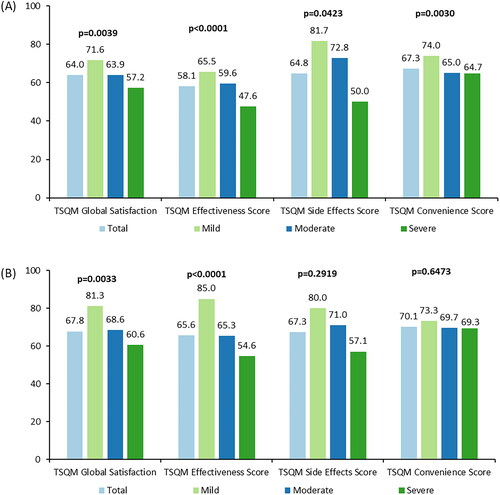
In participants receiving systemic medications, the mean (SD) TSQM global satisfaction, effectiveness, side effects, and convenience scores were 67.8 (23.0), 65.6 (25.2), 67.3 (31.8), and 70.1 (16.4), respectively (). Significant differences were observed in stratification by disease severity for TSQM global satisfaction (p = .0033) and effectiveness (p < .0001) domains. Pairwise comparisons showed significantly higher scores for participants with mild AD for global satisfaction (vs. severe AD; p < .01), and effectiveness (vs. moderate AD; p < .01 and vs. severe AD; p < .001). TSQM side effects and convenience domain scores were not statistically different between the AD severity groups.
AD healthcare visits
Most of the participants visited a dermatologist (n = 133; 71.5%) for AD treatment. Participants most often visited any HCP for AD every three (n = 47; 26.9%) or six months (n = 43; 24.6%); visit frequency increased with disease severity (p = .0045). Most participants (n = 114; 65.1%) received an appointment within two weeks of contacting any HCP and traveled <30 min to reach the HCP’s office (n = 131; 74.9%) ().
Table 4. Healthcare provider visits for atopic dermatitis treatmentTable Footnotea.
AD treatments and expectations
The most used current topical treatments were topical corticosteroid creams or ointments (n = 100; 53.8%) and emollients or moisturizers (n = 90; 48.4%), and the most used current systemic treatments were oral antihistamines (n = 58; 31.2%) and biologics (n = 54; 29.0%). Among topical and systemic treatments, disease severity only impacted the usage of oral antihistamines (p = .0048) and oral or injected corticosteroids (p = .0044) (). Overall, 47.3% of participants reported that they are taking all medications as prescribed.
Table 5. Atopic dermatitis treatmentTable Footnotea.
In total, 38.7% (n = 72) of participants reported that they had declined AD treatment at least once. The most declined topical treatment was topical corticosteroid cream or ointment (n = 27; 37.5%), and the most declined systemic treatments were biologics (n = 25; 34.7%), immunosuppressants (n = 23; 31.9%), and oral or injected corticosteroids (n = 21; 29.2%). Among participants who declined a topical corticosteroid cream or ointment, the proportion of participants with mild AD (66.7%) was significantly higher than those with moderate (23.5%) or severe AD (40.0%; p = .0260). Significant differences were not observed between the AD severity groups for systemic treatments (). The most common reason for declining topical or systemic treatments was the potential for side effects (Appendix 1).
The most common reasons for stopping or changing AD treatment were ‘the treatment did not work’ (n = 126; 67.7%) and ‘side effects of treatment’ (n = 76; 40.9%) (). In terms of improving the current treatment’s characteristics, participants most frequently reported ‘a medication that helps me reduce my symptoms’ and ‘a medication that helps me reduce my flares’ (data not shown).
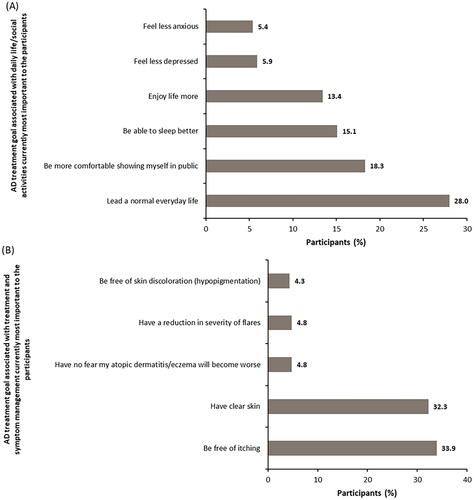
The most important treatment goal associated with daily life/social activities for participants was to ‘Lead a normal everyday life’ (n = 52; 28.0%) (); significant differences were present between the AD severity groups (p = .0008). The most important treatment goals associated with treatment and symptom management for participants were ‘be free of itching’ (n = 63; 33.9%) and ‘have clear skin’ (n = 60; 32.3%) ().
Figure 3. Atopic dermatitis treatment goal associated with daily life/social activities and treatment and symptom management currently most important to participants. (A) AD treatment goals associated with daily life/social activities currently most important to the participants. (B) AD treatment goals associated with treatment and symptom management currently most important to the participants. In figure 3A, n = 186. Other reasons accounting to <5% each were: be comfortable in my sex life (4.3%), be more productive in my everyday life (3.8%), be less of a burden on relatives/friends (1.1%), engage in normal leisure activities (1.1%), be less of a burden in my marriage or partnership (1.1%), be more productive in my working life (0.5%), and have more social contact with people (0.5%).
In figure 3B, n = 186. Other reasons accounting to <4% each were: be free of skin pain (3.2%), have a reduction in frequency of flares (3.2%), have improved predictability of atopic flares from day to day (2.7%), find a clear diagnosis and treatment plan (2.7%), have a treatment that works quickly (2.2%), be less dependent on doctor and clinic visits (1.6%), have fewer of side effects of treatment (1.6%), be free of burning sensation on skin (0.5%), spend less time on treatment (0.5%), use fewer treatments (0.5%), have fewer out-of-pocket treatment expenses (0.5%), and have confidence that therapy will work (0.5%).AD: atopic dermatitis.
Overall, 39.8% (n = 74) participants reported their treatment goals matched their HCP’s treatment goals ‘very closely’, while 16.2% (n = 30) reported their treatment goals matched ‘not very closely’ or ‘not at all’ (). Participants with severe AD were more likely to report mismatched goals (p = .0005).
Discussion
In the current study, we evaluated the humanistic burden of AD, AD treatment satisfaction, and treatment expectations in patients with AD in the US, overall and stratified by disease severity. We observed that greater disease severity decreased QoL and work productivity, increased activity impairment, and decreased treatment satisfaction. The treatment goals of patients with higher disease severity and the perceived goals of HCPs were more likely to not match.
The mean DLQI score in the current study was higher than previous studies, most likely due to the greater proportion of participants with severe AD in this study (26.3%, defined by PO-SCORAD) compared with previous studies (11.0% and 8.1%, defined by the Patient-Oriented Eczema Measure Scale) (Citation4,Citation9). Enrollment was monitored in our study to ensure that ≤25% of the participants approximated mild AD. In addition to the sixfold greater mean DLQI score observed in participants with severe AD compared with mild AD in this study, the stark difference in the proportion of participants with mild and severe AD reporting a ‘very large’ or ‘extremely large’ effect of AD on their lives shows the high burden of severe AD on QoL.
Except for absenteeism, the WPAI scores in the current study were generally comparable to scores reported in two studies evaluating data from the 2013 National Health and Wellness Survey (Citation11,Citation26). Further, participants with moderate or severe AD had 2–4-fold and 5–10-fold higher scores across the WPAI questionnaire domains than those with mild AD, respectively. In the current study, 51.6% of the participants reported some sort of an impact of AD on career and work life; participants with severe AD reported an impact (69.4%) more than those with mild (22.0%) or moderate AD (59.0%). Many participants (59.7%) reported that AD affected their educational activities, relationships, family plans, or leisure activities. These results corroborate prior findings on the heavy lifestyle burden imposed by AD, especially in patients with moderate or severe disease (Citation9).
In the current study, the most-used current systemic treatments were oral antihistamines. However, evidence on efficacy of antihistamines as a part of AD treatment is insufficient (Citation15,Citation27–30). In addition, blocking histamine receptors does not lead to significant improvement in itch or inflammation associated with AD (Citation31). This could be a reason for lower treatment satisfaction among patients with AD. In a recent retrospective study of adult patients with AD, 21.1% of those receiving topical therapy and 30.8% receiving topical + systemic therapy were ‘less than satisfied’ with their current AD treatments (Citation20), suggesting an unmet need in patients using either topical or both topical and systemic treatments. Although this study did not compare TSQM scores of participants based on treatment category, participants on topical therapy scored numerically lower than those on systemic therapy. Moreover, in line with studies from Japan and the US (Citation19,Citation32), the lowest treatment satisfaction was observed in participants with severe AD. These findings emphasize the need for effective treatments in patients with more severe AD. Although new treatments have been recently approved for use (Citation17), their effect in the real-world setting remains to be seen (Citation33).
In the current study, participants commonly stopped or changed AD treatment due to ‘ineffectiveness’ (67.7%) or ‘side effects’ (40.9%). This is consistent with German patients with AD who reported ‘adverse events’ (43.8%) and ‘ineffectiveness’ (22.9%) as the major reasons for treatment discontinuation (Citation34). It is important to note that ‘ineffectiveness’ and ‘side effects’ were the most common reasons for discontinuing or changing treatment across severity levels. Among participants who declined AD treatment (38.7%), ‘fear of potential side effects’ was also the most common reason. This commonality suggests that the patients’ emphasis on disease control and safe medications is unmet by currently available treatments.
In line with a cross-sectional German study (Citation35), the most important treatment goal associated with daily life/social activities for participants in the current study was to ‘Lead a normal everyday life’. In terms of symptom management, ‘be free of itching’ and ‘have clear skin’ were the most important treatment goals in the current and Augustin et al.’s study (Citation35) as itching is the most burdensome symptom (Citation9,Citation36). Although we did not evaluate treatment goals based on disease severity, research shows patients with greater severity have more needs, especially those related to handling adverse effects (Citation35).
When asked about treatment goals, most participants (71.0%) in the current study felt that their treatment goals matched somewhat/very closely with their HCP’s treatment goals; however, greater disease severity was associated with a greater mismatch. For shared decision-making in clinical practice, physicians must encourage and facilitate a discussion to enable patients to elucidate their needs and goals, as well as educate patients about available treatments, including their potential benefits and associated risks. This will help patients and HCPs determine, through shared decision-making, the appropriate individualized therapeutic strategy and consequently achieve the best possible outcomes in the management of AD.
Limitations
Anonymous web-based data collection prevented clinical verification of diagnosis and other clinical data. To overcome this limitation, participants were recruited from four clinical sites, allowing clinicians to confirm the diagnosis in 40% of the sample. Moreover, participants recruited in both modalities had similar characteristics. The study did not include some important confounders such as age, sex, household income, and region, which may also influence the results. The generalizability of the results may be limited due to convenience sampling via the NEA advocacy group and clinical sites. Replicating this research in countries with different healthcare systems, diagnoses, and treatment patterns may reveal similar or varying results. Finally, as participants self-reported the data it might be subject to recall bias (Citation37).
Conclusions
In this real-world cross-sectional study of participants with AD, negative humanistic findings were found across all AD severities but were higher in those with more severe AD. Treatment satisfaction with topical and systemic medications generally decreased with increasing disease severity. Our findings emphasize the need for effective treatments in AD, especially in those with more severe disease.
Acknowledgements
Leo J. Philip Tharappel and Amit Kumar Koushik of Eli Lilly Services India Private Limited, Bengaluru, India provided medical writing and editorial support. Eli Lilly and Company, United States funded support for this assistance.
Disclosure statement
EDB and JC are employed by Evidera, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In their salaried positions, they work with a variety of companies and organizations and are precluded from receiving any payment or honoraria directly from these organizations for services rendered. EJP, ARA, and ZD are employed by and own stock in Eli Lilly and Company. ARA has consulted for Henkel and received the Pfizer Independent Grant for Learning and Change. WSB has received grant funding from Pfizer and advisory board honoraria from Pfizer and Incyte. LB has received advisory board honoraria from Incyte. WSB and LB are salaried employees of the National Eczema Association, which has received grants and sponsorship awards from a variety of industry partners (full list at https://nationaleczema.org/about-nea/corporate-supporters).
Data availability statement
YouGov provided Evidera with de-identified, fully documented datasets. Evidera may provide the datasets generated during and/or analyzed during the current study upon request; contact Elizabeth Bacci at [email protected].
Additional information
Funding
References
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl. 1):1–11.
- Harrop J, Chinn S, Verlato G, et al. Eczema, atopy and allergen exposure in adults: a population-based study. Clin Exp Allergy. 2007;37(4):526–535.
- Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):595–605.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590.
- US Census Bureau. S0101 age and sex 2020. ACS 5-year estimates subject tables; 2020. Available from: https://data.census.gov/cedsci/table?y=2020&tid=ACSST5Y2020.S0101
- Bieber T, Straeter B. Off-label prescriptions for atopic dermatitis in Europe. Allergy. 2015;70(1):6–11.
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol. 2021;184(2):304–309.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol. 2019;181(3):554–565.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274–279.e3.
- Smith Begolka W, Chovatiya R, Thibau IJ, et al. Financial burden of atopic dermatitis out-of-pocket health care expenses in the United States. Dermatitis. 2021;32(1S):S62–S70.
- Eckert L, Gupta S, Amand C, et al. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54–61.e1.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349.
- Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an Expert Panel of the International Eczema Council. J Am Acad Dermatol. 2017;77(4):623–633.
- FDA Approved Drugs. Listings in atopic dermatitis. Falls Church (VA): CenterWatch; 2022 [cited 2022 May 6]. Available from: https://www.centerwatch.com/directories/1067-fda-approved-drugs/topic/760-atopic-dermatitis
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40.
- Wei W, Ghorayeb E, Andria M, et al. A real-world study evaluating adeQUacy of existing systemic treatments for patients with moderate-to-severe atopic dermatitis (QUEST-AD): baseline treatment patterns and unmet needs assessment. Ann Allergy Asthma Immunol. 2019;123(4):381–388.e2.
- Anderson P, Austin J, Lofland JH, et al. Inadequate disease control, treatment dissatisfaction, and quality-of-life impairments among US patients receiving topical therapy for atopic dermatitis. Dermatol Ther. 2021;11(5):1571–1585.
- Bacci E, Rentz A, Correll J, et al. Patient-reported disease burden and unmet therapeutic needs in atopic dermatitis. J Drugs Dermatol. 2021;20(11):1222–1230.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Severity strata for POEM, PO-SCORAD, and DLQI in US adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2018;121(4):464–468.e3.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365.
- Atkinson MJ, Kumar R, Cappelleri JC, et al. Hierarchical construct validity of the Treatment Satisfaction Questionnaire for Medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl. 1):S9–S24.
- Whiteley J, Emir B, Seitzman R, et al. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016;32(10):1645–1651.
- Klein PA, Clark RAF. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135(12):1522–1525.
- Dimson S, Nanayakkara C. Do oral antihistamines stop the itch of atopic dermatitis? Arch Dis Child. 2003;88(9):832–833.
- Herman SM, Vender RB. Antihistamines in the treatment of atopic dermatitis. J Cutan Med Surg. 2003;7(6):467–473.
- Matterne U, Böhmer MM, Weisshaar E, et al. Oral H1 antihistamines as ‘add-on’ therapy to topical treatment for eczema. Cochrane Database Syst Rev. 2019;1(1):CD012167.
- Rukwied R, Lischetzki G, McGlone F, et al. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol. 2000;142(6):1114–1120.
- Nakahara T, Fujita H, Arima K, et al. Treatment satisfaction in atopic dermatitis relates to patient-reported severity: a cross-sectional study. Allergy. 2019;74(6):1179–1181.
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148(4):927–940.
- Pino Lopez J, Kromer C, Herr R, et al. Drug survival rates and reasons for drug discontinuation in patients with atopic dermatitis: a retrospective study of adult outpatients. Eur J Dermatol. 2021;31(2):233–238.
- Augustin M, Langenbruch A, Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152.
- Understanding the Lived Experience of Eczema. The “Voice of the Patient” report on the eczema patient-focused drug development meeting. More Than Skin Deep; 2020.
- Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217.

