Abstract
Background
Upadacitinib is an oral Janus kinase (JAK) 1 inhibitor approved in Japan for moderate-to-severe atopic dermatitis (AD), and it provides a high therapeutic efficacy.
Objectives
We compared the therapeutic effects of upadacitinib on skin rashes of individual anatomical sites, head and neck, upper limbs, lower limbs, and trunk in patients with AD.
Methods
From August 2021 to December 2022, 65 Japanese patients with moderate-to-severe AD (aged ≥ 12 years) were treated with oral once daily upadacitinib 15 mg plus twice daily topical corticosteroids of moderate-to-strongest classes.
Results
The eczema area and severity indexes (EASIs) of individual sites decreased significantly at weeks 4, 12, and 24 compared to those at week 0 in parallel to total (whole body) EASI. The achievement rates of EASI 75 at week 24 and of EASI 90 at week 12 of lower limbs were significantly higher than those of trunk. The percent reductions of EASI of lower limbs at weeks 12 and 24 were significantly higher than those of head and neck and of trunk.
Conclusions
Among the four anatomical sites, the treatment responsiveness to upadacitinib in lower limbs appeared the highest, while those in trunk and in head and neck appeared relatively lower.
Introduction
Upadacitinib is an oral Janus kinase (JAK) 1 inhibitor approved in Japan for moderate-to-severe atopic dermatitis (AD) (Citation1,Citation2). We previously reported the short-term efficacy and safety of upadacitinib for AD in real-world clinical practice (Citation3). We also examined the therapeutic effects of baricitinib, an oral JAK1/JAK2 inhibitor, on the rashes in different anatomical sites and reported lower improvement of rash in head and neck and higher improvement of that in lower limbs (Citation4). Our study also showed that high eczema area and severity index (EASI) of lower limbs before treatment may predict higher percent reduction of total (whole body) EASI at week 12 of baricitinib treatment, while high EASI of head and neck before treatment may predict low percent reduction of total EASI at week 4 (Citation4). On the other hand, the differential effects of upadacitinib on skin rash of individual anatomical sites have not yet been investigated in patients with AD.
We herein compared the therapeutic effects of upadacitinib on skin rash of four individual anatomical sites, head and neck, upper limbs, lower limbs, and trunk in Japanese adult and adolescent patients with moderate-to-severe AD in real-world clinical practice. We also attempted to detect background factors that predict the high improvement of rash in each anatomical site by upadacitinib treatment.
Methods
Study design and data collection
We enrolled patients with moderate-to-severe AD (EASI >7) in this study. The diagnosis of AD was made clinically based on the Japanese Atopic Dermatitis Guidelines 2021 (Citation5). Patients’ sex, age, body mass index (BMI), and disease duration of AD were recorded (). From August 2021 to December 2022, 65 adult and adolescent Japanese patients with AD (aged ≥ 12 years) living in Chiba Prefecture were treated with oral once daily upadacitinib 15 mg plus twice daily topical corticosteroids of moderate-to-strongest classes. Levels of immunoglobulin E (IgE), thymus and activation-regulated chemokine (TARC), lactate dehydrogenase (LDH), and total eosinophil count (TEC) were analyzed before treatment. Total (whole body) EASI, EASI of head and neck, upper limbs, lower limbs, and trunk were analyzed at weeks 0, 4, 12, and 24 of treatment. The medical records above were analyzed retrospectively. This study was conducted based on the Declaration of Helsinki (2004), and was approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital. Patients provided written informed consent. The proportion of patients who achieved EASI 50, EASI 75, EASI 90, or EASI 100 and whose EASI decreased by at least 50%, 75%, 90%, or 100% from baseline, respectively, was calculated at weeks 4, 12, and 24 of treatment.
Table 1. Demographics and baseline characteristics of patients with atopic dermatitis.
Statistical analysis
The Shapiro–Wilk test was used to assess normality. The results are expressed as mean ± standard deviation (SD) for variables with a normal distribution and median and interquartile range for variables with a nonparametric distribution. Differences in the variables at weeks 0, 4, 12, and 24 of treatment were assessed using repeated-measures of analysis for variables with a normal distribution and Friedman’s test for variables with a nonparametric distribution. Post hoc analysis was performed using Bonferroni’s correction. Statistical significance was set at p < .05. Spearman’s correlation coefficient was used to analyze the correlation. Differences between the two groups were analyzed using Student’s t-test for variables with a normal distribution and Mann–Whitney’s U-test for variables with a nonparametric distribution. Fisher’s exact test was used to assess the significance of differences in the frequency distributions. A linear multivariate regression analysis was performed to assess the predictive factors of the high percentage reduction of EASI of each anatomical site at weeks 4, 12, or 24 of upadacitinib treatment. The analyses included only the variables with p values less than .05 in the univariate analyses and were adjusted for age and sex. Variables with a variance inflation factor greater than 10 were excluded to avoid multicollinearity. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical School, Shimotsuke, Japan).
Results
Demographics and baseline characteristics of the patients
Sixty-five Japanese patients with AD (46 males and 19 females) were studied. The presence or absence of prior dupilumab treatment, baseline levels (week 0) of total EASI, EASI of head and neck, upper limbs, lower limbs, and trunk, IgE, TARC, LDH, and TEC are shown in . The proportion of male or adult patients was higher than that of female or adolescent patients, respectively. All the patients manifested IgE values higher than normal levels (>170 IU/mL).
The relations of total EASI or EASIs of individual sites with background factors before treatment
Before treatment, total EASI, head and neck, and trunk EASIs positively correlated with IgE, TARC, LDH, and TEC (). The upper limb and lower limb EASIs positively correlated with IgE, TARC, and LDH. Total EASI, upper limb, lower limb, and trunk EASIs in patients with prior dupilumab treatment were lower than those in patients without, indicating the therapeutic effects of dupilumab.
Table 2. Correlation between baseline background factors and eczema area and severity index for each anatomical site.
Improvement of total EASI and EASIs of individual sites after treatment of upadacitinib plus topical corticosteroids
Total EASI significantly decreased at weeks 4, 12, and 24 compared with baseline levels (week 0) (, Supplemental Table 1). Total EASI at weeks 12 and 24 did not further decrease compared to that at week 4. The EASIs of all individual anatomical sites decreased in manners similar to that of total EASI (, Supplemental Table 1). The percent reduction of total EASI was 76.09%, 75.22%, or 82.13% at weeks 4, 12, or 24, respectively (). The percent reductions of EASI of all the individual sites increased at and after week 4 in manners similar to that of total EASI. The percent reductions of EASI of lower limbs at weeks 12 and 24 were significantly higher than those of head and neck and of trunk ().
Figure 1. The transition of total eczema area and severity index (EASI) and EASIs of individual sites (a), medians of percent reduction of EASI (b), achievement rates of EASI 75 (c), EASI 90 (d), and EASI 100 (e) at weeks 0, 4, 12, or 24 of treatment with upadacitinib plus topical corticosteroids in patients with atopic dermatitis (n = 65). *p < .05 between trunk versus lower limbs and †p < .05 between head and neck versus lower limbs, analyzed by Friedman’s test in (b) or by Fisher’s exact test in (c, d).
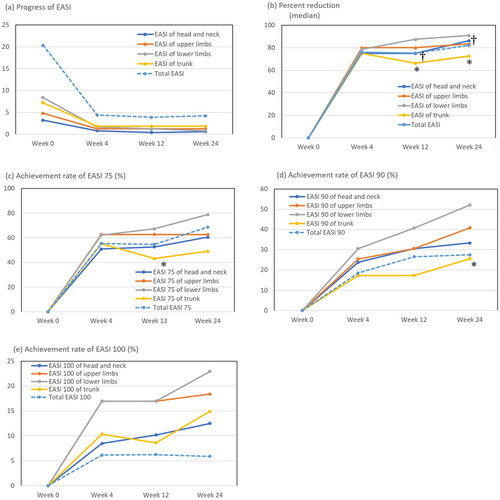
The achievement rates of total EASI 75 and total EASI 90 gradually increased until week 24 () while that of total EASI 100 peaked at week 4 and did not increase thereafter ().The achievement rate of total EASI 75 was 55.38%, 54.69%, or 68.63%, that of total EASI 90 was 18.46%, 26.56%, or 27.45%, and that of total EASI 100 was 6.15%, 6.25%, or 5.88% at weeks 4, 12, or 24, respectively (e)). The achievement rates of EASI 75 or EASI 90 of individual sites increased at and after week 4 in manners similar to those of total EASI () while those of EASI 100 of individual sites gradually increased until week 24 (). The achievement rates of EASI 75, EASI 90, and EASI 100 of lower limbs appeared highest among those of four anatomical sites, while those of trunk or head and neck appeared relatively lower (e)). The achievement rate of EASI 75 of lower limbs at week 12 () and that of EASI 90 of lower limbs at week 24 () were significantly higher than those of trunk. These results indicate that the responsiveness to upadacitinib treatment in the rash of lower limbs appeared highest among the four anatomical sites while that of trunk or head and neck appeared relatively lower.
Background factors that predict the high response of rash on individual anatomical sites to the treatment with upadacitinib plus topical corticosteroids
We then examined background factors that can predict the high response of rash on individual anatomical sites to the treatment with upadacitinib plus topical corticosteroid, as assessed by the percent reduction of EASI of individual sites. In the rash of head and neck, percent reduction of EASI at week 4 or 12 positively correlated with baseline (pretreatment) LDH or age, respectively, while that at week 24 negatively correlated with BMI (). In the rash of upper limbs, percent reductions of EASI at weeks 4 and 12 negatively correlated with baseline IgE (). In the rash of lower limbs, percent reduction of EASI at week 4 was higher in female patients than in male patients, and that at week 12 was higher in patients with prior dupilumab treatment than in patients without (). Percent reduction of EASI of lower limbs at week 4 negatively correlated with baseline IgE and TARC, and that at week 12 negatively correlated with baseline IgE while that at week 24 positively correlated with disease duration and age (). In the rash of trunk, percent reduction of EASI at week 4 was higher in female patients than in male patients (). Percent reduction of trunk EASI at week 4 negatively correlated with baseline IgE while that at week 12 positively correlated with age ().
Table 3. The relations of patients’ background factors with percent reduction of EASI of head of and neck at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 4. The relations of patients’ background factors with percent reduction of EASI of upper limbs at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 5. The relations of patients’ background factors with percent reduction of EASI of lower limbs at weeks 4, 12, or 24 of upadacitinib treatment in patients with atopic dermatitis (n = 65).
Table 6. The relations of patients’ background factors with percent reduction of EASI of trunk at weeks 4, 12, or 24 of upadacitinib treatment for patients with atopic dermatitis (n = 65).
We then aimed to detect the factors predicting high response to upadacitinib treatment in the rash of individual sites. Linear multivariate regression analysis showed that female sex was associated with high percent reduction of EASI of lower limbs at week 4 (), however, failed to detect the association with other background factors. Linear multivariate regression analyses for the percent reduction of EASI of head and neck, upper limbs, and trunk failed to detect any association with background factors (Supplemental Tables 2–4). The results indicate that female sex may predict good improvement of the rash on lower limbs at week 4 by upadacitinib treatment.
Table 7. The predictive factors for the percent reduction of eczema area and severity index (EASI) of lower limbs at weeks 4, 12, or 24 of upadacitinib treatment assessed by linear multivariate regression analysis in patients with atopic dermatitis (n = 65).
Discussion
Baseline total EASI before treatment significantly correlated with baseline levels of all laboratory parameters (IgE, TARC, LDH, and TEC). Some studies reported positive correlation of IgE with total EASI, consistent with our results (Citation6), while other studies reported no correlations of IgE with total EASI (Citation7), possibly due to the difference of patients’ population, age, sex distribution, or race. Baseline TARC and LDH significantly positively correlated with baseline total EASI and EASIs of four anatomical sites. Relatedly, previous reports suggested that TARC is the most reliable indicator reflecting severity of AD, and that LDH could also act as an indicator of severity (Citation8). Previous reports have shown that TEC correlates with the severity of AD (Citation9). Baseline EASI of individual sites mostly correlated with baseline IgE, TARC, LDH, and TEC except for no correlation between baseline TEC and EASIs of upper or lower limbs. This suggests that the values of IgE, TARC, LDH, and TEC might reflect the long-term control of rash on almost all the anatomical sites.
The time-dependency of the transition of EASI of all anatomical sites mostly paralleled that of total EASI, indicating that the improvements of rash on individual sites may similarly contribute to that of whole body rash. However, there was some difference in the responsiveness to upadacitinib treatment dependently on the anatomical sites. The percent reductions of EASI of lower limbs at weeks 12 and 24 of upadacitinib treatment were significantly higher than those of head and neck and of trunk. Relatedly, we have recently reported that the JAK 1/2 inhibitor baricitinib improved EASI of individual anatomical sites with the higher percent reduction in lower limbs than in head and neck at week 12 (Citation4). The post hoc analysis of clinical trial of JAK1 inhibitor abrocitinib (JADE COMPARE) showed that EASI 75 response by abrocitinib 100 mg was achieved at median 30–32 days for lower limbs while at 56–57 days for head and neck, indicating the faster response in lower limbs than in head and neck (Citation10). The skin of head and neck is likely to be exposed to external stimuli such as aeroallergens, sunlight or cosmetics compared to lower limbs. Facial skin is more likely to develop adverse effects of topical corticosteroids, such as telangiectasia or acne. Pathogens such as Malassezia and Demodex are involved in the facial rash in AD patients (Citation11). It is possible that seborrheic dermatitis, contact dermatitis, or rosacea may be related to the head and neck rash in AD patients (Citation11). These complicated features may make head and neck rashes less responsive to upadacitinib treatment.
Further, the head and neck rash in AD patients appeared to be less responsive to dupilumab treatment compared to that of the other anatomical sites, indicating the treatment resistance of the head and neck rash is independent of the types of treatment (Citation12). Malassezia furfur abundantly colonized in the head and neck region may be involved in the pathogenesis of erythema and may contribute to its treatment resistance in AD patients; colonization of Malassezia furfur may disrupt the barrier of corneal layer by reducing the esterified omega-hydroxyacyl-sphingosine content and may induce the expression of vascular endothelial growth factor (VEGF), IL-31, and IL-33 in epidermal keratinocytes and of VEGF receptor, transforming growth factor-β, tumor necrosis factor-α, and IL-1β in dermal endothelial cells, which may promote inflammation, angiogenesis, and tissue remodeling, leading to the generation of erythema (Citation13).
The trunk, mostly covered by clothing, creates an enclosed space that is more likely to cause heat retention, contributing to itching. Patients with AD are associated with sweat retention via the decreased sweating by autonomic imbalances or histamine-induced suppression, or via occlusion of sweat pores by horny plugs; the sweat retention causes skin dryness and heat retention. Patients with AD are also associated with sweat leakage into the surrounding dermis due to the reduced claudin-3 expression in sweat gland/ducts, causing painful itching and maintenance of inflammation due to proteases, histamine, salt, LL-37, or contaminated skin surface antigens in the sweat (Citation14). Regional sweat rate is generally higher in trunk than in lower limbs (Citation15). Thus, the trunk may be the area susceptible to itching or retention of inflammation induced by the sweating impairment in AD patients (Citation14,Citation16), leading to the lower responsiveness to upadacitinib treatment.
The lower limbs are relatively evaded from external stimuli compared to head and neck, and less susceptible to the sweat impairment compared to trunk. These anatomical characteristics of lower limbs might contribute to the higher responsiveness to upadacitinib treatment.
The anatomical site-dependent treatment responsiveness, revealed in upadacitinib treatment, was similarly seen in the other types of treatment for AD; the higher treatment responsiveness in lower limbs was shown in baricitinib treatment (Citation4) and lower responsiveness in head and neck was shown in dupilumab treatment (Citation12). The anatomical site-dependent treatment responsiveness might thus be independent from the types of treatment for AD. The potential differences in microbiome among different anatomical sites might be related to the site-dependent treatment responsiveness. Several studies reported the differences in skin microbiome among different anatomical sites; the α-diversity of skin microbiome in forehead is lower than that in forearm (Citation17). The highest level of 16S rRNA copies of bacteria was present in the axilla among the different body locations, forehead, behind the ear, forearm, foreleg, and axilla (Citation18). Streptococcus was the most common genus present on the forehead and behind the ear while Corynebacterium spp. were predominant in the axilla (Citation18). Malassezia spp. account for 53–80% of all fungi in the different body locations, and the highest numbers were found behind the ear (Citation18). However, except for the abundance of Malassezia in head and neck region, the site-dependent differences in microbiome directly related to the site-dependent treatment responsiveness have not been obtained to date, and should further be detected in future study. The potential differences in resident immune cells among different anatomical sites might also be related to the site-dependent treatment responsiveness in AD patients. However, the anatomical site-dependent differences in the resident immune cells, such as T cells, dendritic cells, macrophages, or mast cells, have not been reported to date. Future studies might reveal anatomical site-specific findings in microbiome and/or resident immune cells directly related to the site-dependent treatment responsiveness, which may provide the mechanisms for the site-dependent treatment responsiveness and guide the development of site-specific treatments for AD.
Linear multivariate regression analysis showed that female sex was associated with the higher percent reduction of EASI of lower limbs at week 4 of upadacitinib treatment. Relatedly, we previously found that female sex was associated with high percent reduction of total EASI at week 12 of upadacitinib treatment (Citation3). The higher treatment response in females than in males may be possibly because female patients might apply higher doses of corticosteroids on the whole body including lower limbs compared to male patients, though we could not precisely compare the topical corticosteroid doses used by female versus male patients in this study. Further studies should examine the corticosteroid doses practically applied at each time point simultaneously with examining the treatment response to upadacitinib, and should clarify the sex difference in the doses.
This study has several limitations. We could not compare the treatment responses to upadacitinib among individual features of rash, erythema, induration/papulation, excoriation, and lichenification. The differential treatment responses among different features of rash should be examined in further studies. Second, this study evaluated only the therapeutic effects of 15 mg/day of upadacitinib, and not of 30 mg/day, which should be examined in further studies. Third, there is a lack of information on the dose and rank of topical corticosteroids used for different anatomical sites. Treatment responsiveness might be affected by the dose and rank of corticosteroids, which might influence the anatomical site-dependent differences in treatment responsiveness. For instance, it is common to apply less potent corticosteroids to the face, which may result in the lower treatment response of the head and neck rash compared to that of the other sites. Therefore, future studies should record the detailed information about the topical corticosteroids for individual anatomical sites.
Conclusions
In conclusion, total EASI significantly decreased at weeks 4, 12, and 24 of upadacitinib treatment compared to baseline levels. The EASIs of individual anatomical sites decreased in response to upadacitinib treatment at and after week 4 in manners similar to those of total EASI; however, the improvement of rash appeared highest in lower limbs while relatively lower in trunk or head and neck. The percent reductions of EASI of lower limbs at weeks 12 and week 24 were significantly higher than those of head and neck and of trunk. The achievement rate of EASI 75 of lower limbs at week 12 and that of EASI 90 at week 24 were significantly higher than those of trunk. Linear multivariate regression analyses showed that female sex was associated with high percent reduction of EASI of lower limbs at week 4 of upadacitinib treatment. Among the four anatomical sites, the treatment response to upadacitinib in lower limbs appeared highest, while that of trunk and of head and neck appeared relatively lower. Female sex may predict high treatment response in the rash of lower limbs at week 4 of upadacitinib treatment.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and February 10, 2022 of approval).
Author contributions
Teppei Hagino mainly organized the manuscript, and Naoko Kanda, Eita Fujimoto, and Hidehisa Saeki revised it. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download Zip (58.5 KB)Disclosure statement
H. S. received a lecture fee and research cost from AbbVie GK. T. H. and N. K. received lecture fees from AbbVie GK.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):1–9.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167.
- Hagino T, Saeki H, Kanda N. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023.
- Saeki H, Ohya Y, Furuta J, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int. 2022;71(4):448–458.
- Chu H, Shin JU, Park CO, et al. Clinical diversity of atopic dermatitis: a review of 5,000 patients at a single institute. Allergy Asthma Immunol Res. 2017;9(2):158–168.
- Stone SP, Muller SA, Gleich GJ. IgE levels in atopic dermatitis. Arch Dermatol. 1973;108(6):806–811.
- Thijs J, Krastev T, Weidinger S, et al. Biomarkers for atopic dermatitis: a systematic review and meta-analysis. Curr Opin Allergy Clin Immunol. 2015;15(5):453–460.
- Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59(6):561–570.
- Alexis A, de Bruin-Weller M, Weidinger S, et al. Rapidity of improvement in signs/symptoms of moderate-to-severe atopic dermatitis by body region with abrocitinib in the phase 3 JADE COMPARE study. Dermatol Ther. 2022;12(3):771–785.
- Darabi K, Hostetler SG, Bechtel MA, et al. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009;60(1):125–136.
- Blauvelt A, Rosmarin D, Bieber T, et al. Improvement of atopic dermatitis with dupilumab occurs equally well across different anatomical regions: data from phase III clinical trials. Br J Dermatol. 2019;181(1):196–197.
- Chu H, Kim SM, Zhang K, et al. Head and neck dermatitis is exacerbated by Malassezia furfur colonization, skin barrier disruption, and immune dysregulation. Front Immunol. 2023;14:1114321.
- Murota H, Yamaga K, Ono E, et al. Sweat in the pathogenesis of atopic dermatitis. Allergol Int. 2018;67(4):455–459.
- Coull NA, West AM, Hodder SG, et al. Body mapping of regional sweat distribution in young and older males. Eur J Appl Physiol. 2021;121(1):109–125.
- Murota H, Yamaga K, Ono E, et al. Why does sweat lead to the development of itch in atopic dermatitis? Exp Dermatol. 2019;28(12):1416–1421.
- Staudinger T, Pipal A, Redl B. Molecular analysis of the prevalent microbiota of human male and female forehead skin compared to forearm skin and the influence of make-up. J Appl Microbiol. 2011;110(6):1381–1389.
- Gao Z, Perez-Perez GI, Chen Y, et al. Quantitation of major human cutaneous bacterial and fungal populations. J Clin Microbiol. 2010;48(10):3575–3581.

