Abstract
Background: Isotretinoin is frequently used for treatment of severe nodulocystic and papulopustular acne, however use is limited by mucocutaneous, ocular, and systemic side effects.
Objective: (1) provide a systematic meta-analysis of ocular side effects during isotretinoin use and their corresponding incidences; (2) provide a narrative summary of ocular side effects during isotretinoin use reported in case reports.
Methods: A systematic database search using predefined search terms was performed in PubMed, EMBASE, and Scopus from inception to 5 March, 2021. Predetermined inclusion and exclusion criteria were used to select included studies. In total, 53 original studies qualified for meta-analysis, and 41 case reports/series qualified for narrative results.
Results: The studies included in the meta-analysis reported incidences of various ocular side effects including dry eye, eye sensitivity, vision changes, and ocular inflammatory conditions. Incidences across studies did vary, leading to considerable heterogeneity. The narrative results summarize more uncommon, but equally important, ocular side effects.
Conclusions: Dry eye is the most commonly reported ocular side effect. Other less common, but more serious, ocular side effects including vision changes can occur. We recommend that isotretinoin prescribers monitor for dry eye. Limitations include the heterogeneity of reported incidences of ocular side effects between studies.
1. Introduction
Isotretinoin, or 13-cis-retinoic acid, was introduced in the United States in 1982 for the treatment of severe nodulocystic and papulopustular acne. The most common indication for use is acne vulgaris, yet it is also prescribed for other dermatologic conditions including rosacea, folliculitis, photoaging and various dermatitides (Citation1). Its primary mechanism of action is to inhibit sebum production, which in turn decreases comedogenesis. Additionally, while it inherently has no antimicrobial properties, it has been shown to decrease skin surface levels of C. acnes, which acts as an opportunistic pathogen in patients with acne vulgaris. A patient taking isotretinoin at a dose of 0.5–1.0 mg/kg/day for six weeks may have a nearly 90% decrease in sebum production (Citation2).
While isotretinoin is a very effective treatment option for patients with recalcitrant acne vulgaris, its clinical use is associated with a variety of adverse events ranging in severity and implications. It is well recognized that the most serious potential adverse event of isotretinoin use is teratogenicity. Yet, there are many other more common side effects, including mucocutaneous effects such as cheilitis and xerosis, and neurologic effects such as headaches (Citation3). There are also several reported ophthalmologic side effects that also range in severity. These include less severe effects such as ocular surface alterations, dry eye diseases, ocular irritation, visual field defects, and abnormalities in dark adaptation. More serious and extremely rare adverse effects include retinal toxicity, corneal opacities, and optic neuritis, which may lead to permanent ocular damage if unrecognized (Citation4,Citation5). These ocular adverse events have been reported within days to years of isotretinoin therapy initiation (Citation6). In some reported cases, these ocular side effects are suspected to be dose dependent and become tolerable with a change in dosing and symptomatic management (Citation2).
Although several reviews exist (Citation1,Citation3–5), to the authors knowledge, there are no systematic reviews specifically analyzing the incidence of ophthalmologic side effects of isotretinoin use. In this study we performed a systematic review and meta-analysis and report the ocular side effects and the corresponding incidences for patients who have taken isotretinoin. In addition, we compiled narrative case reports of isotretinoin use and associated ocular adverse events. Dermatologists and eye care providers may use these summative conclusions to guide clinical decisions regarding need for ocular referrals, and timing of follow-up and therapeutic interventions to monitor and minimize ocular adverse events.
2. Methods
The study was conducted according to the PRISMA guidelines (Citation7) and the review protocol was registered in INPLASY database with an ID of INPLASY202160042.
2.1. Data sources and search strategy
A comprehensive database search using predefined search terms (Figure S1) was performed in PubMed, EMBASE, and Scopus from inception to March 5, 2021. The main keywords used were isotretinoin, 13-cis-retinoic acid, eye, ocular, and ophthalmic. The searches yielded 1,255 articles.
2.2. Study selection and outcomes
All articles were screened independently by 2 of 4 authors for inclusion at each stage of study selection. Any discrepancies between the inclusion/exclusion decisions were resolved through discussion with 2 authors. Selection was performed based on the following inclusion criteria: (1) cohort study, case-control study, randomized control trial (RCT), or case series/case report; (2) participants of all age groups who were taking isotretinoin; (3) reports the occurrence of an ocular side effect; (4) published from 1970 to present. Exclusion criteria consisted of (1) duplicate publication; (2) conference paper, meta-analysis, review; (3) participants taking medication other than isotretinoin; (4) did not report occurrence of ocular side effects or lack thereof; (5) written in a language other than English; (6) did not involve human subjects.
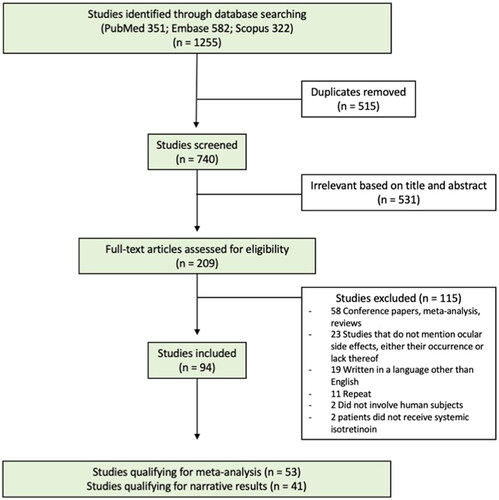
A total of 1,255 studies were identified through the database searches. Following computerized elimination of duplicate studies, 740 studies were screened based on title and abstract and 209 full-text studies were evaluated. A total of 94 studies met al.l inclusion criteria and 115 met exclusion criteria (). Obtained studies were categorized by study type (cohort, case-control, RCT, case series, case report) and then organized into two data-type groups. One group consisted of cohort, case-control, and RCTs to be included in the meta-analysis and the other group consisted of case series and case reports to be included in the narrative results. This resulted in 53 studies qualifying for the meta-analysis and 41 studies qualifying for the narrative results.
2.3. Data extraction
Two authors independently extracted data from the 94 eligible studies. Disagreements were resolved through discussion with two authors. The information extracted differed between the two data-type groups. The following information was extracted from the narrative studies: study design, characteristics of the participants, dosage and duration of isotretinoin use, diagnosis being treated with isotretinoin, ocular side effect description, length of isotretinoin treatment before side effect occurred, length of time side effect lasted, and treatment and/or discontinuation in response to side effect. The following information was extracted from the meta-analysis studies: study design, number and characteristics of the participants, dosage and duration of isotretinoin use, diagnosis being treated with isotretinoin, incidence of ocular side effects, duration of ocular side effect, and participant discontinuation of isotretinoin frequency and reasoning.
2.4. Quality assessment and risk of bias
Two authors independently assessed the quality of evidence of the 53 studies qualifying for the meta-analysis. Disagreements were resolved independently by a third author. The risk of bias was evaluated using the Cochrane Collaboration’s Risk of Bias Assessment Tool (Citation8) for randomized studies and the Cochrane ROBINS-I tool (Citation9) for nonrandomized studies. The overall quality assessment rating is defined by the amount of risk of bias identified in the categories of the tools above. A good rating is defined as meeting all criteria with low risk in all categories and interventions spelled out clearly. Studies were graded ‘fair’ if any or multiple categories were unclear or had low or medium risk of bias. Studies were graded ‘poor’ if any category had a high risk of bias.
2.5. Statistical analysis
Random effects meta-analysis were performed in Stata using ‘metaprop’ command for binary and ‘metan’ commands for continuous outcomes (Citation10). Score confidence intervals and I2 tests of heterogeneity were reported.
3. Results
3.1. Quality assessment and risk of bias evaluation
A total of 53 studies were included in the quality assessment and risk of bias evaluation. The results are reported in Figures S2 and S3.
3.2. Narrative results – case reports and case series
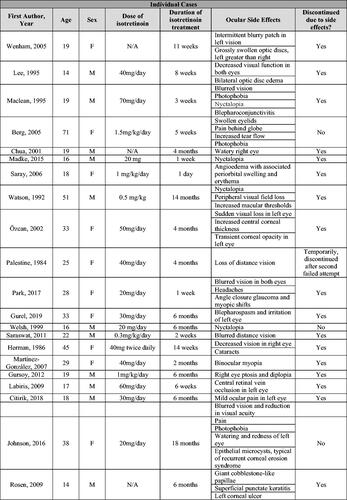
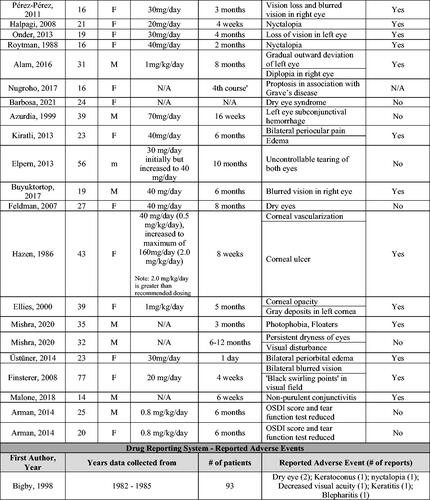
For the narrative results, 41 manuscripts reporting a total of 135 patient cases were reviewed (Citation11–51). Forty of the 41 articles were case reports or case series, which included 42 total patient cases. The additional study was published by Bigby et al. and summarizes cases reported to a drug reporting system from 1982–1985 in which the authors reported 104 suspected adverse events occurring in 93 total patients (Citation51).
From the 40 articles consisting of case reports and case series, the dosages of isotretinoin for the 42 studied patients ranged from 0.5–1.5 mg/kg/day with an average duration of 20.8 weeks of treatment (Citation11–50). These patients had a median age of 23 years (range 14–77). Due to the ocular side effects experienced, 31 of 42 patients discontinued isotretinoin treatment. Bigby et al. did not report on the average dosage of isotretinoin, age of included patients, or discontinuation of treatment (Citation51).
In all 41 articles, the most common ocular symptoms or side effects during isotretinoin use were blurred vision (17/137 cases) and nyctalopia (night blindness) (7/137 cases). In addition, there were 5 cases of dry eye and 4 cases of light sensitivity (Citation11–51). As case reports and case series are more likely to report unusual or unexpected events, more common ocular side effects may be underrepresented.
Details pertaining to each case are summarized in .
Figure 2. Narrative results – table of side effects during isotretinoin treatment from individual cases in case reports and series and adverse drug reporting system.
3.3. Meta-analysis results
A total of 53 studies qualified for meta-analysis of various side effects during isotretinoin treatment. Figure S4 provides a breakdown of the number of patients included per study.
The following 15 studies were excluded from the meta-analysis due to data being non-compatible with other studies. Ersoz et al. (Citation52), Yilmaz et al. (Citation53), Yavuz et al. (Citation54), Yuksel et al. (Citation55), Karadag et al. (Citation56), Ucak et al. (Citation57), Ozyol et al. (Citation58). reported ocular structural changes via changes in the thickness of various layers of the eye. These layers and measurements differed between studies and thus were not included in the final analysis. Hodgkiss-Harlow et al. reported side effects in number of patient months (Citation59). We were unable to determine the number of patients that experienced the side effect and thus this study was excluded from the final analysis. Brown et al. (Citation60), de Queiroga et al. (Citation61), Yeh et al. (Citation62), Fouladgar et al. (Citation63), and Meigel (Citation64) reported ocular side effects unique to their studies and therefore were excluded from the final analysis. Strauss (Citation65) and McLane (Citation66) were excluded because they reported a cumulative number of patients experiencing the given side effect at any time point, unlike the included studies which reported a proportion of patients within a given time interval, and thus were not included in the final analysis.
The goal of this study was to determine accurate incidences of isotretinoin ophthalmologic side effects. We summarize findings in forest plots for both continuous, reported as a mean value, and proportional data (). In general, the reported incidences across studies did vary, leading to considerable heterogeneity. A heterogeneity value of p < 0.05 demonstrates considerable heterogeneity or differences amongst studies. Studies that reported side effects as continuous variables different than other studies were excluded from the forest plot.
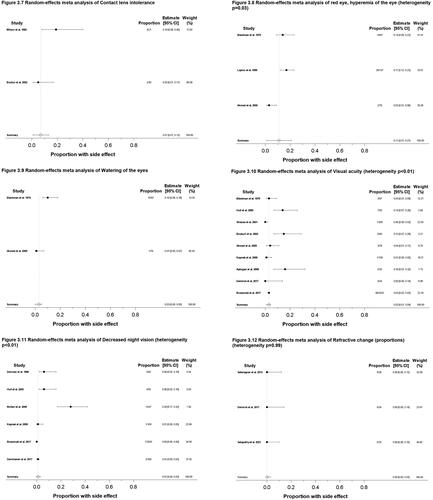
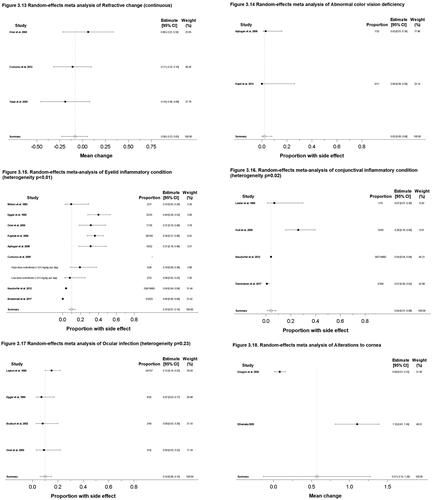
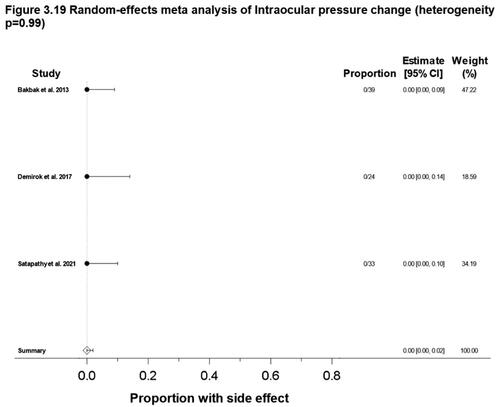
Figure 3. Meta-analysis forest plots for various ocular side effects. (3.1) Dry or irritated eye, proportion; (3.2) Schirmer test scores, mean change; (3.3) TBUT values, mean change; (3.4) TBUT values, proportion; (3.5) OSDI scores, mean change; (3.6) Photophobia, proportion; (3.7) Contact lens intolerance, proportion; (3.8) Red eye/Hyperemia of the eye, proportion; (3.9) Watering of the eyes, proportion; (3.10) Visual acuity change, proportion; (3.11) Decreased night vision, proportion; (3.12) Refractive change, proportion; (3.13) Refractive change, mean change; (3.14) Abnormal color vision deficiency, proportion; (3.15) Eyelid inflammatory condition, proportion; (3.16) Conjunctival inflammatory condition, proportion; (3.17) Ocular infection, proportion; (3.18) Alterations to cornea, mean change; (3.19) Intraocular pressure change, proportion. Random effects meta-analysis were performed in Stata using ‘metaprop’ command for proportions or binary outcomes. Random effects meta-analysis were performed in Stata using ‘metan’ commands for mean change or continuous outcomes.10 Score confidence intervals and I2 tests of heterogeneity were reported. (TBUT: Tear break-up time; OSDI: Ocular surface disease index).
3.3.1. Dry eye
Dry eye is a commonly reported side effect of isotretinoin treatment. This side effect can be reported utilizing a variety of different terminologies including complaints of dry or irritated eye as well as objective measures such as change in Schirmer eye test score, change in tear break-up time (TBUT), and change in ocular surface disease index (OSDI) score. Other common complaints of ocular discomfort such as photophobia, contact lens intolerance, red eye, hyperemia of the eyes, and watering of the eyes are often associated with dry eye. Below are the results of the forest plots for these reported categories ().
Sixteen studies (19,906 total patients) reported proportions of patients experiencing dry or irritated eye (Citation1,Citation67–81). The overall reported incidence is 27% (95% CI: 23%, 31%) with heterogeneity of p < 0.01 ().
Eight studies (299 total patients) reported continuous Schirmer eye test scores from before and during isotretinoin treatment () (Citation81–88). Isotretinoin treatment was associated with an overall −2.38 change in Schirmer eye test scores (95% CI: −4.15, −0.62), with heterogeneity of p < 0.01. One study (Oner 2005 (Citation81)) was an outlier for Schirmer test scores, with a large negative mean change of −8.79 (95% CI: −11.10, −6.48). This was reviewed by our authors; despite the fact that the results are largely different, no considerable dissimilarities in methodology were identified as compared to other included studies.
Seven studies (289 total patients) reported continuous TBUT values from before and during isotretinoin treatment () (Citation81,Citation83–86,Citation88,Citation89). Isotretinoin treatment was associated with an overall −3.60 change (95% CI: −5.40, −1.79) in TBUT values, with heterogeneity of p < 0.01.
Three studies (130 total patients) reported TBUT values as a proportional change from before and during isotretinoin treatment () (Citation77,Citation80,Citation81). The overall reported incidence is 36% (95% CI: 0%, 73%) with heterogeneity of p < 0.01.
Four studies (185 total patients) reported the continuous OSDI scores from before and during isotretinoin treatment () (Citation72,Citation84,Citation86,Citation88). Isotretinoin treatment was associated with an overall 21.48 increase in OSDI scores (95% CI: 12.38, 30.57), with heterogeneity of p < 0.01.
Other complaints of ocular discomfort that may serve as surrogates for dry eye include photophobia, contact lens intolerance, red eye, hyperemia of the eyes, and watering of the eyes. Five studies (1182 total patients) reported the proportion of patients experiencing photophobia (Citation70,Citation74,Citation78,Citation90,Citation91). The overall reported incidence is 9% (95% CI: 0%, 19%) with heterogeneity of p < 0.01 (). Two studies (61 total patients) reported the proportion of patients experiencing contact lens intolerance (Citation75,Citation80). The overall reported incidence is 7% (95% CI: 1%, 13%) (). 3 studies (332 total patients) reported the proportion of patients experiencing red eye or hyperemia of the eye (Citation67,Citation74,Citation92). The overall reported incidence is 11% (95% CI: 1%, 21%) with heterogeneity of p = 0.03. (). Two studies (175 total patients) reported the proportion of patients experiencing watering of the eyes (Citation74,Citation92). The overall reported incidence is 3% (95% CI: 0%, 5%) (). Overall, in 9 unique studies reporting on 1478 total patients, the overall reported incidence of eye sensitivity side effects ranged from 3–11%.
3.3.2. Vision changes
Vision changes included visual acuity change, refractive vision changes, nyctalopia, and abnormal color vision. Nine studies (4684 total patients) reported the proportion of patients experiencing visual acuity changes (Citation1,Citation68,Citation74,Citation78–80,Citation92–94). The overall reported incidence is 3% (95% CI: 0%, 4%) with heterogeneity of p < 0.01 (). Six studies (4211 total patients) reported the proportion of patients experiencing nyctalopia (Citation1,Citation70,Citation78,Citation93,Citation95,Citation96). The overall reported incidence is 1% (95% CI: 0%, 3%) with heterogeneity of p < 0.01 (). Three studies (85 total patients) reported the proportion of patients experiencing refractive vision change (Citation94,Citation97,Citation98). The overall reported incidence is 0% (95% CI: 0%, 3%) with heterogeneity of p = 0.99 (). Three studies (111 total patients) reported the continuous refractive vision changes from before and during isotretinoin treatment (Citation81,Citation99,Citation100). There was an overall −0.08 change (95% CI: −0.23, 0.05) after initiation of isotretinoin treatment (). Two studies (43 total patients) reported the proportion of patients experiencing abnormal color vision deficiency (Citation68,Citation101). The overall reported incidence is 2% (95% CI: 0%, 8%) (). Overall, in unique 18 studies reporting on 5265 total patients, the overall reported incidence of vision changes ranged from 0–3%, with 3 studies reporting a 0.08 decrease in refractive vision associated with isotretinoin.
3.3.3. Eyelid inflammatory condition
Eyelid inflammatory conditions were defined as blepharitis, blepharoconjunctivitis, scales and collarettes on the lids, meibomitis, chalazion, and hordeolum. Eight studies (18,501 patients) reported the proportion of patients experiencing eyelid inflammatory conditions (Citation1,Citation68,Citation69,Citation75,Citation77,Citation81,Citation89,Citation93). The overall reported incidence is 10% (95% CI: 7%, 14%) with a heterogeneity of p < 0.01 ().
3.3.4. Conjunctival inflammatory condition
Conjunctival inflammatory conditions were defined as conjunctivitis and conjunctival papillary hypertrophy. Four studies (15,047 patients) reported the proportion of patients experiencing conjunctival inflammatory conditions (Citation69,Citation70,Citation78,Citation90). The overall reported incidence is 4% (95% CI: 1%, 8%) with a heterogeneity of p = 0.02 ().
3.3.5. Ocular infection
Ocular infection was defined as bacterial conjunctivitis and eye infection. Four studies (287 patients) reported the proportion of patients experiencing ocular infection (Citation67,Citation77,Citation80,Citation81). The overall reported incidence is 10% (95% CI: 6%, 15%) with heterogeneity of p = 0.23 ().
3.3.6. Alterations to the cornea
Alterations to the cornea were defined as disruption in corneal surface via change in corneal fluorescein staining score/ocular staining score. Two studies (75 patients) reported the continuous corneal fluorescein staining score/ocular staining score change () (Citation72,Citation88). There was an overall +0.57 change (95% CI: −0.14, 1.28) after initiation of isotretinoin treatment.
3.3.7. Intraocular pressure change
Three studies (96 patients) reported the proportion of patients intraocular pressure change (Citation94,Citation98,Citation102). The overall reported incidence is 0% (95% CI: 0%, 2%) with heterogeneity of p = 0.99 ().
4. Discussion
Isotretinoin is an effective therapy for many dermatological conditions (Citation1). Due to the drug’s inhibition of sebum production, the meibomian glands are particularly affected (Citation103). Decreased meibomian oil production primarily manifests as dry eye. We found that dry eye is the most commonly reported ocular side effect, as elucidated by patient reports and measurable ocular tests. While decreased meibomian oil production typically manifests as dry eye, many other downstream ocular side effects can occur as well. These include ocular inflammatory conditions, ocular infection, and symptoms of eye sensitivity such as light intolerance, contact lens intolerance, red eye, and watering of the eyes (Citation69,Citation75,Citation77,Citation81).
It is important for clinicians to address and educate patients about the potential ophthalmologic side effects of isotretinoin. Specifically, we suggest that patients be educated on potential ocular side effects such as dry eye and signs of more serious ocular complications, such as vision alteration. Clinicians should evaluate for these symptoms while patients are taking isotretinoin. Therapeutic interventions to address dry eye include lubricating eye drops, avoidance of contact lens use, and avoidance of prolonged screen time while using isotretinoin (Citation63). Lubricating eye drops increase tear film stability, reduce surface stress, and increase quality of life for patients. For patients experiencing persistent moderate to severe dry eye or any signs of serious ocular complications, we suggest referral for an ophthalmological examination (Citation104).
Current recommendations for patients taking isotretinoin include follow-up appointments with a dermatologist every 30 days (Citation2). While there are recommendations for laboratory monitoring for serum lipid and liver abnormalities with isotretinoin use, similar guidance for monitoring ocular manifestations does not exist. Unfortunately, subjective symptoms of dry eye do not always correlate with objective clinical findings. Patients with only mild symptoms may have severe dry eye and vision-threatening ocular complications (Citation104). Given this, we recommend assessment of ocular symptoms at every follow-up visit, with a moderate threshold to refer for ophthalmological assessment to assess severity of ocular manifestations.
The high degree of heterogeneity between studies is most likely due to the varied study designs; there was variability in the populations studied and the way data were reported. It may also demonstrate that there is considerable variability in the literature regarding the reported incidence of ocular side effects during isotretinoin use. Some of the investigations were performed by dermatology teams and others by ophthalmology, and their methods of reporting symptoms or measures of ocular side effects often varied. For example, only two studies reported the incidence of contact lens intolerance and only three studies reported proportions of patients experiencing intraocular pressure change. In other situations, some studies reported TBUT as a pooled net change over time amongst all patients while others reported the proportion of patients who experienced a TBUT change. There were other symptom groups with similar differences in reporting technique. For this reason, separate analyses were performed for each unique reporting method. Overall, the study is limited by the high degree of heterogeneity of reported incidences of ocular side effects between studies and limited numbers of studies for some of the analyses.
The narrative results highlight more uncommon, but equally important ocular side effects. For example, the reported incidence of nyctalopia (decreased night vision) and visual acuity changes in the meta-analysis were lower than other ocular side effects. However, blurred vision and night blindness were the most common side effects mentioned in case reports and series of patients taking systemic isotretinoin (). Screening for these side effects among patients taking isotretinoin may be warranted. Providers should be aware that rare yet serious ophthalmic side effects such as ocular ulcers and cataracts can occur, and may warrant regular ophthalmologic examination for patients with preexisting ocular conditions. Among the reported cases, side effects led to discontinuation of isotretinoin in 72% of patients.
5. Conclusions
Ocular side effects of systemic isotretinoin can have serious consequences if not recognized and appropriately treated. Dry eye is the most common side effect and symptom, and can lead to more serious side effects such as ocular inflammatory conditions and ocular infection. It is recommended that prescribers of isotretinoin monitor for dry eye. Patients with dry eye may benefit from lubricating eye drops and ophthalmologic examination to determine the severity of ocular disease.
Supplemental Material
Download Zip (54.4 KB)Acknowledgments
The review authors gratefully acknowledge University at Michigan informaticist Kathryn Vanderboll.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in public domain resources (Citation1,Citation11–102). These data were derived from the following resources available in the public domain: PubMed, EMBASE, and Scopus from inception to March 5, 2021.
Additional information
Funding
References
- Brzezinski P, Borowska K, Chiriac A, et al. Adverse effects of isotretinoin: a large, retrospective review. Dermatol Ther. 2017;30(4):1.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1(3):162–12.
- Vallerand IA, Lewinson RT, Farris MS, et al. Efficacy and adverse events of oral isotretinoin for acne: a systematic review. Br J Dermatol. 2018;178(1):76–85.
- Ruiz-Lozano RE, Hernández-Camarena JC, Garza-Garza LA, et al. Isotretinoin and the eye: a review for the dermatologist. Dermatol Ther. 2020;33(6):e14029.
- Charakida A, Mouser PE, Chu AC. Safety and side effects of the acne drug, oral isotretinoin. Expert Opin Drug Saf. 2004;3(2):119–129.
- Fraunfelder FT, Fraunfelder FW, Edwards R. Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol. 2001;132(3):299–305.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med. 2009;6(7):e1000097.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(2):d5928–d5928.
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
- Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10.
- Gursoy H, Cakmak I, Yildirim N, et al. Presumed isotretinoin-induced, concomitant autoimmune thyroid disease and ocular myasthenia gravis: a case report. Case Rep Dermatol. 2012;4(3):256–260.
- Lee AG. Pseudotumor cerebri after treatment with tetracycline and isotretinoin for acne. Cutis. 1995;55(3):165–168.
- Maclean H, Wright M, Choi D, et al. Abnormal night vision with isotretinoin therapy for acne [3]. Clin Exp Dermatol. 1995;20(1):86.
- Nugroho J, Schweiger B. Isotretinoin as a possible environmental trigger to autoimmunity in genetically susceptible patients. Case Rep Pediatr. 2017;2017:4207656.
- Finsterer J. Enhanced ocular isotretinoin toxicity in mitochondrial disorder. South Med J. 2008;101(6):664–665.
- Berg G, Andersson T, Sjödell L, et al. Development of severe thyroid-associated ophthalmopathy in a patient with disseminated thyroid cancer treated with recombinant human thyrotropin/radioiodine and retinoic acid. Thyroid. 2005;15(12):1389–1394.
- Üstüner P. Angioedema due to systemic isotretinoin therapy. Case Rep Dermatol Med. 2014;2014:595914.
- Hazen P, Carney J, Langston R, et al. Corneal effect of isotretinoin: possible exacerbation of corneal neovascularization in a patient with the keratitis, ichthyosis, deafness (“KID”) syndrome. J Am Acad Dermatol. 1986;14(1):141–142.
- Buyuktortop N, Onaran Z, Özkal F, et al. Central serous chorioretinopathy probably associated with isotretinoin in a keratoconus patient. Can J Ophthalmol. 2018;53(4):e162–e165.
- Pérez-Pérez L, García-Gavín J, Allegue F, et al. Optic neuritis probably induced by isotretinoin. Actas Dermosifiliogr. 2012;103(9):843–844.
- Saray Y, Seçkin D. Angioedema and urticaria due to isotretinoin therapy [13]. J Eur Acad Dermatol Venereol. 2006;20(1):118–120.
- Gurel G, Sacmaci H. Blepharospasm induced by systemic isotretinoin. Akad Acil Tip Olgu Sunumlari Derg. 2019;10(2):33–35.
- Mishra KK, Scholey JE, Daftari IK, et al. Oral isotretinoin and topical retinoid use in a series of young patients with ocular melanoma. Am J Ophthalmol Case Rep. 2020;19:100787.
- Feldman RJ, Maize JC. Multiple keratoacanthomas in a young woman: report of a case emphasizing medical management and a review of the spectrum of multiple keratoacanthomas. Int J Dermatol. 2007;46(1):77–79.
- Kiratli H, Dikmetaş O. Bilateral lacrimal gland enlargement associated with isotretinoin treatment. Ophthalmic Plast Reconstr Surg. 2013;29(6):e156–e157.
- Saraswat A. Sudden irreversible worsening of myopia with isotretinoin treatment. Indian J Dermatol Venereol Leprol. 2011;77(5):611–612.
- Wenham CJ, Clarke I, Shun Shin GA. Isotretinoin-related optic disc swelling. Br J Hosp Med. 2005;66(11):644–645.
- Herman DC, Dyer J. Anterior subcapsular cataracts as a possible adverse ocular reaction to isotretinoin. Am J Ophthalmol. 1987;103(2):236–237.
- Madke B, Prasad K, Kar S. Isotretinoin-induced night blindness. Indian J Dermatol. 2015;60(4):424.
- Palestine AG. Transient acute myopia resulting from isotretinoin (accutane) therapy. Ann Ophthalmol. 1984;16(7):660–662.
- Alam MS, Agarwal S. Presumed isotretinoin-induced extraocular myopathy. J Pharmacol Pharmacother. 2016;7(4):187–189.
- Rosen E, Raz J, Segev F. Giant cobblestone-like papillae during isotretinoin therapy. Ocul Immunol Inflamm. 2009;17(5):312–313.
- Halpagi P, Grigg J, Klistorner A, et al. Night blindness following low-dose isotretinoin. J Eur Acad Dermatol Venereol. 2008;22(7):893–894.
- Azurdia RM, Sharpe GR. Isotretinoin treatment for acne vulgaris and its cutaneous and ocular side-effects. Br J Dermatol. 1999;141(5):947.
- Citirik M, Tekin K. Excessive serous retinal detachment during the use of isotretinoin. Int Ophthalmol. 2018;38(2):763–766.
- Malone C. Isotretinoin induced granulomatosis with polyangitis. J Hosp Med. 2018;13(4):Supplement 1.
- Arman A, Demirseren DD, Akoglu G. Tolerance to systemic isotretinoin therapy in two patients using highly wettable contact lenses. Case Rep Ophthalmol Med. 2014;2014:452462.
- Labiris G, Katsanos A, Karapetsa M, et al. Association between isotretinoin use and Central retinal vein occlusion in an adolescent with minor predisposition for thrombotic incidents: a case report. J Med Case Reports. 2009;3(1):58.
- Johnson M, O’Colmain U, Affleck A. Recurrent corneal erosion syndrome associated with oral isotretinoin: a cautionary tale. Clin Exp Dermatol. 2016;41(5):564.
- Park YM, Lee TE. Isotretinoin-induced angle closure and myopic shift. J Glaucoma. 2017;26(11):e252–e254.
- Barbosa AP, Oliveira FR, da Rocha FJ, et al. Lacrimal gland atrophy and dry eye related to isotretinoin, androgen, and prolactin: differential diagnosis for Sjögren’s syndrome. Arq Bras Oftalmol. 2021;84(1):78–82.
- Roytman M, Frumkin A, Bohn TG. Pseudotumor cerebri caused by isotretinoin. Cutis. 1988;42(5):399–400.
- Martínez-González MC, García-Silva J, Sánchez MA, et al. Acute myopia while on oral isotretinoin treatment. J Eur Acad Dermatol Venereol. 2007;21(7):977–978.
- Elpern DJ. Isotretinoin and one patient’s teary eyes: “please listen or I’ll cry. Int J Dermatol. 2013;52(6):757–758.
- Chua WC, Martin PA, Kourt G. Watery eye: a new side-effect of isotretinoin therapy. Eye . 2001;15(Pt 1):115–116.
- Welsh BM, Smith AL, Elder JE, et al. Night blindness precipitated by isotretinoin in the setting of hypovitaminosis A. Australas J Dermatol. 1999;40(4):208–210.
- Özcan AA, Soylu M. Transient corneal opacity after use of oral isotretinoin. Ann Med Sci. 2002;11(2–4):71–72.
- Ellies P, Dighiero P, Legeais JM, et al. Persistent corneal opacity after oral isotretinoin therapy for acne. Cornea. 2000;19(2):238–239.
- Watson NJ, Hutchinson CH. Rod and cone malfunction as a manifestation of isotretinoin therapy. J Dermatolog Treat. 1992;3(4):205–207.
- Onder HI, Turan H, Kilic AC, et al. Premacular hemorrhage due to isotretinoin use. Cutan Ocul Toxicol. 2013;32(2):170–172.
- Bigby M, Stern RS. Adverse reactions to isotretinoin. A report from the adverse drug reaction reporting system. J Am Acad Dermatol. 1988;18(3):543–552.
- Ersoz MG, Gunes IB, Hazar L, et al. Ocular surface epithelial thickness changes with SD-OCT in patients treated with oral isotretinoin. Int Eye Sci. 2018;18(1):7–11.
- Yılmaz U, Küçük E, Koç Ç, et al. Investigation of the effects of systemic isotretinoin treatment on retinal nerve fiber layer and macula. J Dermatolog Treat. 2017;28(4):314–317.
- Yavuz C, Ozcimen M. An evaluation of peripapillar choroidal thickness in patients receiving systemic isotretinoin treatment. Cutan Ocul Toxicol. 2019;38(1):25–28.
- Yuksel N, Ozer MD, Akcay E, et al. Reduced Central corneal thickness in patients with isotretinoin treatment. Cutan Ocul Toxicol. 2015;34(4):318–321.
- Karadag O, Kocamaz M, Dastan M, et al. Assessment of macular choroidal thickness, Central macular thickness and retinal nerve fiber layer in patients receiving oral isotretinoin treatment. Cutan Ocul Toxicol. 2020;39(3):233–236.
- Ucak H, Aykut V, Ozturk S, et al. Effect of oral isotretinoin treatment on retinal nerve fiber layer thickness. J Cutan Med Surg. 2014;18(4):236–242.
- Ozyol P, Ozyol E, Yildirim FE. Remodeling of cornea with isotretinoin treatment. Eye Contact Lens. 2020;47(6):366–371.
- Hodgkiss-Harlow CJ, Eichenfield LF, Dohil MA. Effective monitoring of isotretinoin safety in a pediatric dermatology population: a novel “patient symptom survey” approach. J Am Acad Dermatol. 2011;65(3):517–524.
- Brown RD, Grattan CE. Visual toxicity of synthetic retinoids. Br J Ophthalmol. 1989;73(4):286–288.
- de Queiroga IBW, Vieira LA, de Nadai Barros J, et al. Conjunctival impression cytology changes induced by oral isotretinoin. Cornea. 2009;28(9 PG-1009-13):1009–1013.
- Yeh TN, Lin MC. Isotretinoin study: ocular surface comparison between exposed and unexposed groups. Investig Ophthalmol Vis Sci. 2016;57(12):2856.
- Fouladgar N, Khabazkhoob M, Hanifnia AR, et al. Evaluation of the effects of isotretinoin for treatment of acne on corneal sensitivity. J Curr Ophthalmol. 2018;30(4):326–329.
- Wn M. How safe is oral isotretinoin? Dermatology. 1997;195:22–28.
- Strauss JS, Gottlieb AB, Jones T, et al. Concomitant administration of vitamin E does not change the side effects of isotretinoin as used in acne vulgaris: a randomized trial. J Am Acad Dermatol. 2000;43(5 Pt 1):777–784.
- McLane J. Analysis of common side effects of isotretinoin. J Am Acad Dermatol. 2001;45(5):S188–S194.
- Layton AM, Cunliffe WJ. Ocular problems in contact lens wearers and non-contact lens wearers on isotretinoin – practical guidelines. J Dermatolog Treat. 1990;1(5):247–249.
- Aydogan K, Turan OF, Onart S, et al. Neurological and neurophysiological effects of oral isotretinoin: a prospective investigation using auditory and visual evoked potentials. Eur J Dermatol. 2008;18(6):642–646.
- Neudorfer M, Goldshtein I, Shamai-Lubovitz O, et al. Ocular adverse effects of systemic treatment with isotretinoin. Arch Dermatol. 2012;148(7):803–808.
- Demirseren DD, Kilinc F, Emre S, et al. The weeks and the cumulative doses of the first adverse events related to oral isotretinoin in acne patients: analysis of 300 patients. J Dermatolog Treat. 2017;28(4):309–313.
- Mirnezami M, Rahimi H. Is oral omega-3 effective in reducing mucocutaneous side effects of isotretinoin in patients with acne vulgaris? Dermatol Res Pract. 2018;2018:1–4.
- Düzgün E, Özkur E. The effect of oral isotretinoin therapy on meibomian gland morphology and dry eye tests. J Dermatolog Treat. 2022;33(2):762–768.
- Hafeez L, Khan AN, Aslam A, et al. Comparison of safety and efficacy of low dose isotretinoin versus the conventional dosing regime in the treatment of acne vulgaris. J Pakistan Assoc Dermatologists. 2020;30(3):423–427.
- Blackman HJ, Peck GL, Olsen TG, et al. Blepharoconjunctivitis: a side effect of 13-cis-retinoic acid therapy for dermatologic diseases. Ophthalmology. 1979;86(5):753–759.
- Milson J, Jones DH, King K, et al. Ophthalmological effects of 13-cis-retinoic acid therapy for acne vulgaris. Br J Dermatol. 1982;107(4):491.
- Clamon G, Chabot GG, Valeriote F, et al. Phase I study and pharmacokinetics of weekly high-dose 13-cis-retinoic acid. Cancer Res. 1985;45(4):1874–1878.
- Egger SF, Huber-Spitzy V, Böhler K, et al. Ocular side effects associated with 13-cis-retinoic acid therapy for acne vulgaris: clinical features, alterations of tearfilm and conjunctival flora. Acta Ophthalmol Scand. 1995;73(4):355–357.
- Hull PR, Demkiw-Bartel C. Isotretinoin use in acne: prospective evaluation of adverse events. J Cutan Med Surg. 2000;4(2):66–70.
- Strauss JS, Leyden JJ, Lucky AW. Safety of a new micronized formulation of isotretinoin in patients with severe recalcitrant nodular acne: a randomized trial comparing micronized isotretinoin with standard isotretinoin. J Am Acad Dermatol. 2001;45(2):196–207.
- Bozkurt B, Irkeç MT, Atakan N, et al. Lacrimal function and ocular complications in patients treated with systemic isotretinoin. Eur J Ophthalmol. 2002;12(3):173–176.
- Oner A, Ferahbas A, Karakucuk S, et al. Ocular side effects associated with systemic isotretinoin. J Toxicol – Cutan Ocul Toxicol. 2005;23(3):189–195.
- Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland morphology and tear osmolarity: changes with accutane therapy. Cornea. 1991;10(4):286–290.
- Aragona P, Cannavò SP, Borgia F, et al. Utility of studying the ocular surface in patients with acne vulgaris treated with oral isotretinoin: a randomized controlled trial. Br J Dermatol. 2005;152(3):576–578.
- Karalezli A, Borazan M, Altinors DD, et al. Conjunctival impression cytology, ocular surface, and tear-film changes in patients treated with systemic isotretinoin. Cornea. 2009;28(1):46–50.
- Yildirim Y, Olcucu O, Agca A, et al. Evaluation of corneal topography and biomechanical parameters after use of systemic isotretinoin in acne vulgaris. J Ophthalmol. 2014;2014:701361.
- Caglar C, Senel E, Sabancilar E, et al. Reduced ocular surface disease index (OSDI) scores in patients with isotretinoin treatment. Int Ophthalmol. 2017;37(1):197–202.
- Polat M, Kükner Ş. The effect of oral isotretinoin on visual contrast sensitivity and amount of lacrimation in patients with acne vulgaris. Cutan Ocul Toxicol. 2017;36(1):35–38.
- Elhamaky TR. Efficacy of omega-3 fatty acids and punctal plugs in the prevention of isotretinoin-associated ocular surface disease. Eur J Ophthalmol. 2020;31(5):2339–2345.
- Cumurcu T, Sezer E, Kilic R, et al. Comparison of dose-related ocular side effects during systemic isotretinoin administration. Eur J Ophthalmol. 2009;19(2):196–200.
- Lester RS, Schachter GD, Light MJ. Isotretinoin and tetracycline in the management of severe nodulocystic acne. Int J Dermatol. 1985;24(4):252–257.
- Goulden V, Layton AM, Cunliffe WJ. Long-term safety of isotretinoin as a treatment for acne vulgaris. Br J Dermatol. 1994;131(3):360–363.
- Ahmed I, Wahid Z, Nasreen S. Adverse effects of systemic isotretinoin therapy: a study of 78 patients. J Pak Assoc Dermatolog. 2005;15(3):242–246.
- Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18(6):576–580.
- Demirok G, Topalak Y, Gündüz Ö, et al. The long-term effect of oral isotretinoin therapy on macula ganglion cell complex thickness. Cutan Ocul Toxicol. 2017;36(3):259–262.
- Denman S, Weleber R, Hanifin JM, et al. Abnormal night vision and altered dark adaptometry in patients treated with isotretinoin for acne. J Am Acad Dermatol. 1986;14(4):692–693.
- Mollan SP, Woodcock M, Siddiqi R, et al. Does use of isotretinoin rule out a career in flying? Br J Ophthalmol. 2006;90(8):957–959.
- Sekeryapan B, Dılek N, Oner V, et al. Retinal nerve fiber layer and ganglion cell layer thickness in patients receiving systemic isotretinoin therapy. Int Ophthalmol. 2013;33(5):481–484.
- Satapathy J, Panigrahi PK, Kar BR. Effect of oral isotretinoin on retinal nerve fibre layer and ganglion cell layer thickness. Int J Pharm Res. 2021;13(1):3427–3432.
- Cumurcu T, Sener S, Ozsoy E, et al. Changes in anterior chamber parameters with the pentacam rotating scheimpflug and axial length measurements by ultrasound in patients who use isotretinoin. Curr Eye Res. 2012;37(5):395–398.
- Yasar E, Gurlevik U, Kemeriz F, et al. Effect of isotretinoin on myopia and axial length: a pilot study. Cutan Ocul Toxicol. 2020;39(4):385–388.
- Kaptı BH, Aslan G, Yavruoğlu MA. Evaluation of retinal nerve fiber layer changes with oral isotretinoin treatment. Ophthalmol Ther. 2013;2(1):19–23.
- Bakbak B, Gedik S, Koktekir BE, et al. Structural and functional assessment in patients treated with systemic isotretinoin using optical coherence tomography and frequency-doubling technology perimetry. Neuroophthalmology. 2013;37(3):1100–103.
- Moy A, McNamara NA, Lin MC. Effects of isotretinoin on meibomian glands. Optom Vis Sci. 2015;92(9):925–930.
- Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81.