Abstract
Background: Limited information exists on the risk of adverse events (AEs) attributed to methotrexate (MTX) and biologics for the treatment of psoriasis/psoriatic arthritis (PsA/PsO) in heterogeneous clinical practice and beyond the duration of clinical trials.
Methods: An observational study of 6294 adults with incident PsA/PsO who initiated MTX or biologics in Stockholm from 2006-2021 was conducted. The risk of kidney, liver, hematological, serious infectious, and major gastrointestinal AEs was quantified and compared between therapies using incidence rates, absolute risks, and adjusted hazard ratios (HRs) from propensity-score weighted Cox regression.
Results: Median follow-up was 4.3 (2–7) years. Users of MTX had a higher risk of anemia (HR 1.79 [95% CI, 1.48–2.16]), particularly mild-moderate anemias (1.93;1.49–2.50), and mild (1.46;1.03–2.06) and moderate-severe liver AEs (2.22;1.19–4.15) compared to biologics. Chronic kidney disease incidence did not differ between therapies (affecting 1.5% of the population in 5 years; HR:1.03;0.48–2.22). Acute kidney injury, serious infections, and major gastrointestinal AEs showed low absolute risks and no clinically meaningful differences between both therapies.
Conclusion: The use of MTX for psoriasis patients in routine care was associated with a higher risk of anemia and liver AEs than biologics, but similar risks of kidney, serious infections, and major gastrointestinal AEs.
Introduction
Low-dose methotrexate (MTX) and biologic agents are the main systemic treatment options for managing psoriasis/psoriatic arthritis (PsO/PsA) (Citation1–4). The latest national clinical guidelines suggest MTX/Adalimumab as first-line therapy for moderate to severe PsO/PsA (Citation5). Concerns for adverse events (AEs) may limit their use. Both treatments have been linked to hematologic, malignant, gastrointestinal, infectious, mucocutaneous, renal, and musculoskeletal AEs (Citation6,Citation7). Most of the available information on the incidence of AEs comes from randomized controlled trials (RCTs). Many of these RCTs, however, may have been too small with an average number of participants of 171(range 50–517) for MTX trials (Citation6) and 487(range 64-1881) for biologics trials (Citation8). They may have also been of short duration: MTX trials had a median duration of 6(IQR 5.5–12) (Citation6) months, and biologics trials 3(3-4) (Citation8,Citation9) months. This makes it difficult to quantify the absolute risks of these outcomes, which tend to be rare or like kidney AEs, occur over many years. This together with the trial”s inclusion and exclusion criteria as well as their strict monitoring protocols may have underestimated AE occurrence (Citation8, Citation10). Patients and clinical practice in routine care are more heterogeneous, potentially affected by lower treatment adherence, lesser monitoring at the clinic, and the presence of other comorbid conditions or ongoing medications that may potentiate AEs. Indeed, observational studies report somewhat higher risks of selected AEs associated with these therapies (Citation11–13). An identified limitation in these studies is the reliance on issued clinical diagnoses to define AEs. In clinical practice, most AEs are identified by laboratory tests (i.e., creatinine, transaminases, hemoglobin), and only those severe enough to deserve issuing a diagnosis are identified by claims.
The objective of this study was to comprehensively quantify and compare the occurrence of potential AEs associated with the initiation of MTX or biologic treatment among patients with PsO/PsA managed in routine care. We used complete administrative healthcare data as well as laboratory testing in the population of the region of Stockholm, Sweden.
Materials and methods
Data source
The study comes from SCREAM, a project in Stockholm, Sweden (Citation14) that collects laboratory tests and healthcare utilization data from residents in the region between 2006–2021. The data includes demographics, prescribed drugs, kidney replacement therapy outcomes, diagnoses, and vital status and is linked to administrative databases using personal identification numbers. The study has complete information without any loss to follow-up from 1997 to the end of 2021.
Study population and study design
We identified adults (≥18 years) residing in Stockholm, diagnosed with PsO/PsA between 2006–2021, and who started treatment with MTX or biologics for the first time. Patients who started therapy with a combination of strategies (e.g., biologics plus MTX), or with comorbid inflammatory comorbidities, such as rheumatoid arthritis, Crohn”s disease, ulcerative colitis, and ankylosing spondylitis, were excluded. However, patients diagnosed with PsA were still included (Citation15) (Table S1 and Figure S1). Patients under 18 years old were also excluded. The index date and start of follow-up was the date of treatment initiation.
Exposure and covariates
The study exposure was initiation of treatment with MTX or biologics. MTX included both oral and subcutaneous formulations. Biologics included tumor necrosis factor- α inhibitors (TNF-αi) and IL-inhibitors medications approved for PsO/PsA (details and definitions in ).
Table 1. Baseline characteristics of PsO/PsA patients initiating biologics or methotrexate treatment.
Study covariates were derived at index date and included demographics (age, sex), calendar year, psoriasis type, comorbidities, other ongoing pharmacological treatments, and laboratory assessment of estimated glomerular filtration rate (eGFR), C-reactive protein, hemoglobin, and liver transaminases (Table S2). eGFR was calculated from plasma creatinine measurements and with the 2009 CKD-EPI equation without correction for race (Citation16). Baseline laboratory values were defined as the closest measurement performed in the 12 months preceding the index date.
Outcomes
We quantified the occurrence of the most relevant AEs reported in the literature in the context of psoriasis (Citation6,Citation7). These included kidneys (CKD progression and acute kidney injury), hematologic (i.e., anemia including aplastic anemia), infectious, hepatic (mild liver events and severe liver events), gastrointestinal, and cutaneous (i.e., severe allergic hypersensitivity reactions) AEs. Table S3 provides details and definitions of the AEs considered, detected by both clinical diagnoses issued and abnormal laboratory values. For instance, mild liver AEs were defined as an elevation of liver enzymes between 2 to 5 times the upper limit of normal liver transaminases from national reference laboratories. Moderate-severe liver AEs included large elevation of liver enzymes or claim data diagnoses and procedure codes of relevant hepatic events (chronic hepatitis, liver fibrosis, liver cirrhosis, liver failure, or unplanned hospital admissions with liver-related primary diagnoses). We considered two complementary definitions of CKD: a claims-based outcome, defined by the presence of a diagnosis of CKD, accepted referral to a nephrologist, or start of kidney replacement therapy; and a laboratory-based outcome, defined by clinically relevant losses in the kidney function (Citation17). The reason is that CKD awareness is low, and most patients with laboratory-assessed CKD lack a clinical diagnosis (Citation18).
We followed patients until the outcome of interest, or until a censoring event, which included treatment discontinuation (defined as the absence of treatment for at least 90 after the end of supply from the last filled prescription), initiation of treatment combinations, treatment cross-overs, emigration, death, or the end of follow-up (December 2021). However, for the outcome of CKD progression, we followed patients until the event, emigration, death, or the end of follow-up given that potential nephrotoxic effects of past medication exposure may contribute to its decline over many years.
Statistical analyses
Continuous variables are presented as mean ± SD or median with interquartile range, and categorical variables as number and percentage. Because differences between patient characteristics may determine the assignment to one or another medication, we used inverse probability of treatment weighting (IPTW) to minimize this confounding by indication (Citation19). IPTW estimates the probability of initiating MTX versus biologics as a function of baseline study covariates, and up-weights or down-weights populations that are similar or dissimilar between groups, respectively. Weights were stabilized by adding the marginal probability of the received treatment to the numerator of the weights. Standardized mean differences were calculated to evaluate the balance of covariates between treatment arms before and after weighting.
We calculated incidence rates per 1000 person-years with 95% CIs for each adverse event. Weighted Cox proportional hazards regression was used to estimate HRs and 95% CIs for the likelihood of an outcome in the MTX vs biologics group. To compare the cumulative incidence and absolute risk of each outcome over time with the Nelson-Aalen IPTW-weighted estimator (Citation20). Weighted Nelson-Aalen curves were also plotted to compare cumulative incidences of adverse events between treatment groups, with confidence intervals obtained by robust variance estimation. Finally, we calculated the numbers needed to harm (NNH) to observe an excess safety event. NNH estimates the number of subjects that would be required to use MTX, rather than biologics, to observe 1 excess safety outcome.
There was no missing data for study covariates, except laboratory tests, which were not ordered in routine care and, therefore, could not be missing at random. Thus, missing data on covariates of interest were categorized as “missing”. Lastly, to investigate potential differential outcome ascertainment due to differences in the frequency of laboratory testing between the biologics and MTX groups, we calculated and compared the rates of testing of these biomarkers during follow-up in each group. All analyses were performed using R version 4.0.5.
Results
Patient characteristics
We identified 6294 eligible patients who initiated MTX or biologics treatment for PsO/PsA in Stockholm during 2006–2021(Figure S1). Most patients (86%) started with MTX, while 14% started with biologics. Biologic therapy was initiated by younger patients (mean age 46 vs 51 years) with slightly higher mean eGFR (). Adalimumab was the most prescribed biologic (46%). After propensity score weighting, all baseline characteristics achieved a good balance (Table S4). Median follow-up was 58 months for MTX and 36 months for biologics, and the median time on treatment was 9 and 14 months for MTX and biologics, respectively. In the MTX group, treatment discontinuation (32%) or add-on biologic treatment (21%) were the main reasons for censoring, while in the biologics group, the main reason was reaching the end of the study period (Table S5).
Occurrence of adverse drug effects
shows incidence rates per 1000 person-years for clinically detected potential AEs. In general, patients on MTX experienced numerically higher incidence rates of kidney, liver, and mild-moderate anemia events than patients on biologics. Conversely, patients on biologics experienced numerically higher incidence rates of major gastrointestinal AEs. Events of severe allergic hypersensitivity were rare, and rates of detected anemia overall or moderate-severe infections were similar.
Table 2. Follow-up time, number of events, incidence rates and relative risk for the association between biologics vs. methotrexate and study outcomes.
The IPTW-adjusted HRs for each of the composite adverse event safety outcomes for MTX compared with biologics are presented in . Absolute risks, absolute risk difference, and NNH (i.e., to observe an excess safety event) for each of the safety events estimated in the IPTW population at 1, 3, 5, and 10 years after treatment are presented in and Figure S2. NNH results estimate how many persons would need to be treated with an MTX vs biologics to observe 1 excess adverse event.
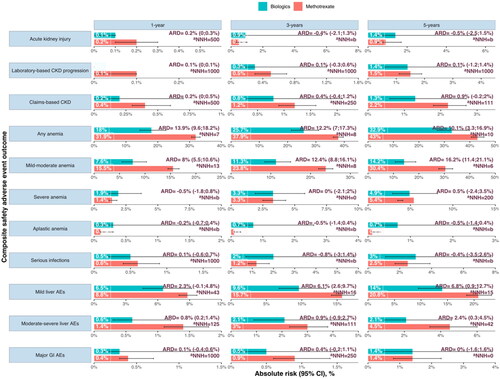
Figure 1. Absolute Risk, absolute risk difference (ARD), and estimated number needed to harm (NNH) for each composite adverse event safety outcome in PsO/PsA patient initiating methotrexate and biologics at 1, 3, and 5 years of therapy. The Nelson- Aalen estimator derived absolute risk estimates from cumulative incidence curves. Risk differences are shown per 1000 persons and rounded to the nearest integer.
aNNH can be interpreted as the number of subjects that would be required to start methotrexate (and not biologics) to observe 1 excess adverse event.
Abbreviations: CKD: chronic kidney disease; ARD: absolute risk difference; NNH: number needed to harm; b) Indicates that a negative number is needed to harm, arising when methotrexate has lower adverse event risks than biologics.
Analyses were adjusted for: age, sex, eGFR, psoriasis type, hypertension, diabetes, myocardial infarction, heart failure, atrial fibrillation, liver disease, mild infections (6 months prior), moderate-severe infections (6 months prior), history of cancer, CKD diagnosis, AKI (6 months prior), topical treatment (corticosteroids, topical calcineurin inhibitors), other systemic Pso/PsA treatment (sulfasalazine, acitretin, leflunomide, apremilast, dimethyl fumarate, cyclosporine), other medications (ACEi/ARB, beta-blocker, Lipid-lowering, anti-diabetics, systemic glucocorticoids, antimicrobials, NSAID), hs-CRP, Hb, AST, ALT and year of treatment initiation.
Kidney-related adverse events
In the “as-treated” analysis, 3 patients in the biologics group and 16 in the MTX group had AKI. The absolute risk of AKI was higher in the MTX group [0.2% (95%CI 0.1–0.3%)] than in biologics [0.1% (0–0.1%)] in the first year of treatment, with an absolute risk difference of 0.2% (0–0.3%) (). The adjusted hazard ratio for AKI was 0.53 [CI, 0.1–2.88] compared to biologics ().
For the laboratory-based CKD progression outcome, we used 102,860 subsequent routine outpatient measurements of eGFR in an intention-to-treat analysis. The 5-year and 10-year absolute risk of the laboratory-based CKD progression outcome was 1.4% (0.6–3.3%) and 4.6% (1.9–11.2%) in the biologics arm, and 1.5% (1.1–1.9%) and 5.6% (4.6–6.8%) in the MTX arm. This corresponds to an absolute risk difference of 0.1% (−1.2–1.4%) and 2.2% (−0.2–4.6%), respectively ( and Figure S2). NNH suggests that if 1000 people were treated with MTX vs biologics for 5 years, 1 extra CKD event would be observed, reduced to 100 after 10 years of treatment (). The relative risk of laboratory-based CKD progression risk was similar for MTX users (HR, 1.03 [CI, 0.48–2.22]) compared with biologics users ( and ).
Figure 2. Weighted cumulative incidence plots for the selected adverse event occurring during the use of biologics or methotrexate in routinely cared patients with PsO/PsA. Shaded areas represent 95% confidence intervals.
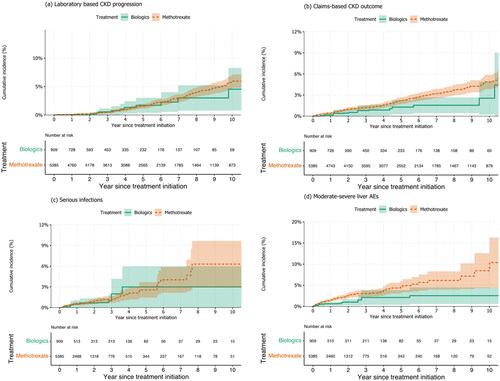
During a mean follow-up time of 4.5 (SD 3.9) years, 154 composite of claims-based CKD outcomes occurred among MTX users, and 13 kidney composite events among biologics users. This corresponds to incidence rates of 5.2(4.4–6.1) and 3.8(2.0–6.5) per 1000 person-years, respectively (). The 5-year and 10-year absolute risk of the composite kidney outcome was 1.3% (0.6–2.7%) and 2.5% (1.0–6.1%) in the biologics arm, and 2.2% (1.8–2.7%) and 4.8% (3.9–5.8%) in the MTX arm. This corresponds to an absolute risk difference of 0.9% (−0.2; 2.0%) and 2.2% (−0.2–4.6%), respectively ( and S2). The adjusted hazard ratio for the kidney composite outcome was 1.06 [CI, 0.53–2.14] (), and the weighted cumulative incidence curve is shown in Figure 3b, which shows a late separation. Similarly,
Hematological adverse events
MTX use was associated with a higher risk of anemia compared to biologics, with an absolute risk difference of 13.9% (9.6–18.2%) and 12.2% (7.0–17.3%) at 1-year and 3-year follow-up, respectively (). Approximately 7 people would need to be treated with MTX for one year to observe one additional anemia event. The weighted cumulative incidence curve clearly separated the two groups in the first three years of follow-up (Figure S3(a)). The relative rate of anemia events was 79% higher for MTX users compared to biologics ().
With regard to anemia severity, MTX use was associated with a higher risk of mild-moderate anemia compared to biologics, with an HR of 1.93 (95% CI 1.49-2.5) (), and absolute risk differences of 8% (95% CI 5.5–10.6%) at 1-year, 12.4% (95% CI 8.8–16.1%) at 3-year, and 8% (95% CI 11.4–21.1%) at 5-year follow-up, with an NNH of 13, 8, and 6, respectively (). Conversely, there was no significant difference in the risk of severe anemia between MTX and biologics at any follow-up time point, with an absolute risk difference of −0.5% (−1.8–0.8%) at 1-year follow-up, 0.0% (−2.1–2.0%) at 3-year follow-up, and 0.5% (−2.4–3.5%) at 5-year follow-up, and a HR of 0.67 (0.45-1.01). The weighted cumulative incidence curve clearly separated the MTX and biologics groups in the first five years of follow-up for mild-moderate anemia (Figure S3(b)). However, there was no significant difference in the risk of severe anemia between the two groups based on the weighted cumulative incidence curve (Figure S3(c)).
The risk of aplastic anemia was similar between the two treatments (HR, 0.38 [CI, 0.11–1.29]).
Serious infections
For MTX, the most common types of serious infection (in order of frequency) were pneumonia, complicated UTI, and sepsis (Table S6). The absolute risk for serious infections was higher for biologics 3.0% [(1.1–7.7%)] than MTX 2.5% [(1.7–3.9%)] (). We did not find a materially different rate of overall serious infection among users of MTX users compared to biologics (HR, 0.89 [CI, 0.39–2.02]; and ).
Liver-related adverse events
The relative rate of mild liver AEs was 47% higher for MTX users (HR, 1.47 [CI, 1.14-1.88]) compared with biologics (). The cumulative incidence curves show early separation between the groups in the early years of follow-up (Figure S2c). The absolute risk difference increases continually from 6.1% (2.6-9.7%); NNH = 16 at 3 years to 13.1% (5.8-20.3%); NNH = 8 at 10 years ( and Figure S1).
Severe hepatic pathologies were more common in the MTX group [liver fibrosis (n = 10), liver failure (n = 4)] versus none in the biologic group (Table S6). The incidence of a composite outcome of serious liver AE was higher for MTX with an absolute risk difference of 5.6% (1.6–9.6%) at 10 years (). Compared to biologics, the relative rates of severe liver AEs for MTX were 115% higher (HR 2.15[1.16–4.0]) ( and ).
Other adverse events
The relative rates of major gastrointestinal adverse outcomes (HR 0.58 [0.24–1.42]) also did not differ between treatment groups ( and ). Two patients in the MTX group had severe allergic hypersensitivity reactions, corresponding to an incidence rate of 0.2 (0.0-0.8) per 1000 person-years and none in biologics.
Supporting analyses
With exception of the rate of blood count testing which is specific to MTX monitoring, testing rates for creatinine, hemoglobin, and liver transaminases measurements were similar during observation in both groups (Table S8), suggesting that detection biases inferred by differential laboratory testing between groups may be small.
Discussion
In this observational study of over 6000 psoriasis patients in Stockholm, we followed new users of MTX and biologics for potential adverse effects for a duration of 4.3 years. MTX users had a higher risk of anemia and liver AEs, but the risk of CKD progression was low and similar to biologics. Other adverse effects had low occurrence and did not differ between treatments. The study has strengths such as a large sample size, long follow-up, careful design, and robustness across sensitivity analyses. The study used real-world patients from a country with universal tax-funded healthcare, minimizing selection bias from disparate access to healthcare.
Myelotoxicity is a potential acute adverse effect of MTX treatment, with anemia, thrombocytopenia, and leukopenia previously reported in 3–10% of patients during the 1980s and 1990s (Citation21–23). In agreement with a more contemporary report (Citation4), we observed a higher rate of anemia in MTX users, especially during the first year of treatment and indicating acute onset. As a clinical application, this first year of therapy may be the moment where more attention should be paid to blood cell count monitoring. Biologic users had lower absolute risks of anemia, but the risks were still clinically relevant. We are unable to identify reports evaluating myelotoxicity associated with biologics, and this AE does not seem to be explicitly reported in pivotal clinical trials (Citation8,Citation24,Citation25). However, various case reports and postulated mechanisms suggest hematologic AEs for anti-TNF therapy (Citation26–29).
Hepatotoxicity has been also reported in connection to systemic psoriasis agents, and guidelines recommend regular monitoring of transaminases. In line with preceding evidence (Citation30–33), we observed a higher risk of liver AEs with MTX use compared to biologics. More recently, abnormalities in liver function tests have been described with anti-TNF drugs such as infliximab, adalimumab, certolizumab, and etanercept (Citation34–37). Previous longitudinal studies on hepatotoxicity in psoriasis are limited to a short follow-up and rely on administrative codes, which tend to identify only a fraction of presumably more severe events. Thus our 10 years of patient trajectories as well as the capture of liver markers should be seen as study strengths.
The concern for potential nephrotoxicity with MTX has led to guidelines recommending regular kidney function monitoring. Although high-dose MTX in oncology (Citation38) or rheumatoid arthritis (Citation39–41) has been associated with nephrotoxicity, our study found low absolute risks with no differences between therapies, likely due to the use of low-dose MTX and the generally younger age and low comorbidity profile of psoriasis patients. In a recent trial of persons with CVD (Citation42) low-dose MTX has been reported to be safe and well tolerated in mild to moderate kidney disease with even suggestion of preservation of eGFR decline compared with placebo, possibly attributed to MTX”s anti-inflammatory effects. A strength of our study compared to previous observational evidence (Citation43,Citation44) is the evaluation of the trajectory of eGFR slopes from all routine creatinine tests (Citation45), as opposed to relying on a single eGFR test or threshold, which are sensitive to detection bias, and misclassification. Because confounding is inherent to observational studies, our study cannot provide definite evidence on renal safety for low-dose MTX in psoriasis. Well-designed prospective studies are still needed to definitively establish the renal safety of low-dose MTX in psoriasis.
While we did not find differences in the rate of serious infections, our study found a slightly higher absolute risk for biologics compared to MTX at long-term follow-up. Evidence from previous studies (Citation46,Citation47) and meta-analyses (Citation48,Citation49) is conflicting, and the observed slight elevation in long-term infection risks may be affected by residual confounding. When putting this evidence in context, we interpret that it is possible for infection risks to be low and not different between therapies and that our observation of slight elevation in long-term risks may be affected by residual confounding. Similarly, while the absolute risk of major GI AEs was higher for biologics users, the relative risk did not differ, suggesting that comorbidities or other medications of biologic users may be a more likely cause of such AEs.
Our study complements evidence from clinical trials with observations from the more heterogeneous clinical practice, with patients not subjected to inclusion/exclusion criteria and having long-term treatments. However, we are subjected to confounding inherent to observational studies. Our study also complements evidence from psoriasis registries; while ours may be a more inclusive/complete population, we had however lesser information on clinical covariates typically recorded in registries, such as disease severity which may modify AEs risks. Additional limitations include the fact that we can only quantify events that were detected by laboratory testing or that resulted in a clinical diagnosis. We did not directly consider the use of folic acid or variations in the dosage or route of administration of MTX (Citation50). However, Sweden has uniform prescribing practice, with MTX in PsO/PsA given once weekly and folic acid given concomitant days after MTX administration. Unlike in trials, laboratory tests in our study were performed as part of routine healthcare per indication and at variable rates of monitoring rather than at predetermined intervals. Nevertheless, our supporting analyses suggested similar testing frequency between the groups, and we thus believe that our findings are unlikely to be explained by differential outcome ascertainment. Finally, these findings related to the region of Stockholm during 2006–2021. Extrapolation to other regions, countries, or periods should be done with caution.
To conclude, we provide observational evidence on higher absolute and relative risks of hematologic and liver AEs associated with MTX use versus biologics in the management of psoriasis. Conversely, we observed low absolute and relative risks of infections, gastrointestinal, AKI or CKD progression events that did not differ between therapies. These finding have clinical implications by demonstrating the rarity of AEs that could not be well-quantified in trials and have long been a concern (such as kidney complications attributed to low-dose MTX) that may have limited the use of these therapies. Thus, our risk estimates may inform the choice of treatments and motivate discussions with patients about potential risks in the shared decision process of psoriasis management. Because many AEs in our study were identified by abnormal laboratory tests, our results support current guideline recommendations to monitor these tests at least once annually.
Author contributions
F.M. and J.J.C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: F.M., J.J.C., and J.L Acquisition, analysis, or interpretation of data: F.M., JJC., A.K., J.L and L.S. Statistical analysis: F.M. and J.J.C Drafting of the article: All authors, Critical revision of the article for important intellectual content: All authors. Supervision: J.J.C.
Supplemental Material
Download PDF (1 MB)Ethical statement
The Regional Ethical Review Board in Stockholm approved the study.
Disclosure statement
J.J.C. received institutional funding from AstraZeneca, Astellas, Amgen, and ViforPharma outside this study. AK received consultation and advisory board membership from Janssen-Cilag AB, Novartis, Amgen, AbbVie. LS is an employee of Amgen. JL served paid speaker for Janssen-Cilag AB, Novartis, AbbVie and Galderma. FM had no disclosures to report.
Data availability statement
The SCREAM project contains sensitive personal data that as per GDPR regulations cannot be publicly shared. We however welcome collaborative project proposals that abide to GDPR, national and institutional regulations for data sharing and data access. For enquiries, please contact [email protected].
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sparks JA, Barbhaiya M, Karlson EW, et al. Investigating methotrexate toxicity within a randomized double-blinded, placebo-controlled trial: rationale and design of the cardiovascular inflammation reduction Trial-Adverse events (CIRT-AE) study. Semin Arthritis Rheum. 2017;47(1):1–9.
- Warren RB, Weatherhead SC, Smith CH, et al. British association of dermatologists” guidelines for the safe and effective prescribing of methotrexate for skin disease 2016. Br J Dermatol. 2016;175(1):23–44.
- Swedish Society for Dermatology and Venereology (SSDV). Treatment recommendations for systemic treatment of psoriasis. Accessed Jan 2023. https://ssdv.se/images/SSDVs_behandlingsrekommendationer_for_systemisk_behandli_ng_av_psoriasis_2023-03-16.pdf.
- No DJ, Inkeles MS, Amin M, et al. Drug survival of biologic treatments in psoriasis: a systematic review. J Dermatolog Treat. 2018;29(5):460–466.
- Swedish Society for Dermatology and Venereology (SSDV). treatment recommendations for systemic treatment of psoriasis. Accessed Jan 2023. https://ssdv.se/images/SSDVs_behandlingsrekommendationer_for_systemisk_behandli_ng_av_psoriasis.pdf.
- West J, Ogston S, Foerster J. Safety and efficacy of methotrexate in psoriasis: a meta-analysis of published trials. PLOS One. 2016;11(5):e0153740.
- Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol. 2018;45(3):279–286.
- Afach S, Chaimani A, Evrenoglou T, et al. Meta-analysis results do not reflect the real safety of biologics in psoriasis. Br J Dermatol. 2021;184(3):415–424.
- Ruyssen-Witrand A, Perry R, Watkins C, et al. Efficacy and safety of biologics in psoriatic arthritis: a systematic literature review and network meta-analysis. RMD Open. 2020;6(1):e001117.
- Yiu ZZN, Exton LS, Jabbar-Lopez Z, et al. Risk of serious infections in patients with psoriasis on biologic therapies: a systematic review and meta-analysis. J Invest Dermatol. 2016;136(8):1584–1591.
- Daudén E, Carretero G, Rivera R, et al. Long-term safety of nine systemic medications for psoriasis: a cohort study using the Spanish registry of adverse events for biological therapy in dermatological diseases (BIOBADADERM) registry. J Am Acad Dermatol. 2020;83(1):139–150.
- Ogdie A, Grewal SK, Noe MH, et al. Risk of incident liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis: a population-based study. J Invest Dermatol. 2018;138(4):760–767.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155(10):1142–1152.
- Carrero JJ, Elinder CG. The Stockholm CREAtinine measurements (SCREAM) project: fostering improvements in chronic kidney disease care. J Intern Med. 2022;291(3):254–268.
- The National Board of Health and Welfare. National guidelines for psoriasis care: Support for governance and management. 2019. date access: Jan 2023. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2019-3-11.pdf.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612.
- Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the US food and drug administration and European medicines agency. Am J Kidney Dis. 2020;75(1):84–104.
- Carrero JJ, Fu EL, Vestergaard SV, et al. Defining measures of kidney function in observational studies using routine health care data: methodological and reporting considerations. Kidney Int. 2023;103(1):53–69.
- Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15(1):14–20.
- Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34(6):585–612.
- Hanrahan PS, Scrivens GA, Russell AS. Prospective long term follow-up of methotrexate therapy in rheumatoid arthritis: toxicity, efficacy and radiological progression. Br J Rheumatol. 1989;28(2):147–153.
- Buchbinder R, Hall S, Sambrook PN, et al. Methotrexate therapy in rheumatoid arthritis: a life table review of 587 patients treated in community practice. J Rheumatol. 1993;20(4):639–644.
- Ohosone Y, Okano Y, Kameda H[, et al. Toxicity of low-dose methotrexate in rheumatoid arthritis–clinical characteristics in patients with MTX-induced pancytopenia and interstitial pneumonitis. Ryumachi. 1997;37(1):16–23.
- Shear NH, Betts KA, Soliman AM, et al. Comparative safety and benefit-risk profile of biologics and oral treatment for moderate-to-severe plaque psoriasis: a network meta-analysis of clinical trial data. J Am Acad Dermatol. 2021;85(3):572–581.
- Xu S, Gao X, Deng J, et al. Comparative efficacy and safety of biologics in moderate to severe plaque psoriasis: a multiple-treatments meta-analysis. J Dtsch Dermatol Ges. 2021;19(1):47–56.
- Falsetti L, Sampaolesi M, Riccomi F, et al. Adalimumab as a potential cause of drug-induced thrombocytopaenic microangiopathy. BMJ Case Rep. 2020;13(3):e233526.
- Cepeda J, Liedke C, Patnaik A, et al. Development of thrombotic thrombocytopenic purpura in association with the monoclonal antibody, golimumab, used to treat rheumatoid arthritis, in a case with literature review. JCR: j Clin Rheumatol. 2018;24(4):229–231.
- Baysal M, Ümit EG, Saritaş F, et al. Drug induced thrombotic microangiopathy with certolizumab pegol. Balkan Med J. 2018;35(5):398–399.
- Harada Y, Yamamoto H, Sato M, et al. Autoimmune hemolytic anemia during adalimumab treatment for plaque psoriasis. Intern Med. 2015;54(9):1103–1104.
- Solomon DH, Glynn RJ, Karlson EW, et al. Adverse effects of low-dose methotrexate. Ann Intern Med. 2020;172(6):369–380.
- Munera-Campos M, Vilar-Alejo J, Rivera R, et al. The risk of hepatic adverse events of systemic medications for psoriasis: a prospective cohort study using the BIOBADADERM registry. J Dermatolog Treat. 2022;33(4):2110–2117.
- Shetty A, Cho W, Alazawi W, et al. Methotrexate hepatotoxicity and the impact of nonalcoholic fatty liver disease. Am J Med Sci. 2017;354(2):172–181.
- Gelfand JM, Wan J, Zhang H, et al. Risk of liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis receiving methotrexate: a population-based study. J Am Acad Dermatol. 2021;84(6):1636–1643.
- Ghabril M, Bonkovsky HL, Kum C, et al. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11(5):558–564.e3.
- Lopetuso LR, Mocci G, Marzo M, et al. Harmful effects and potential benefits of anti-tumor necrosis factor (TNF)-α on the liver. IJMS. 2018;19(8):2199.
- Gisondi P, Cazzaniga S, Chimenti S, et al. Metabolic abnormalities associated with initiation of systemic treatment for psoriasis: evidence from the Italian Psocare Registry. J Eur Acad Dermatol Venereol. 2013;27(1):e30–e41.
- Llamas-Velasco M, Concha-Garzón MJ, García-Diez A, et al. Liver injury in psoriasis patients receiving Ustekinumab: a retrospective study of 44 patients treated in the clinical practice setting. Actas Dermo-Sifiliográficas (English Edition). 2015;106(6):470–476.
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703.
- Schiff MH, Whelton A. Renal toxicity associated with disease-modifying antirheumatic drugs used for the treatment of rheumatoid arthritis. Semin Arthritis Rheum. 2000;30(3):196–208.
- Kremer JM, Petrillo GF, Hamilton RA. Pharmacokinetics and renal function in patients with rheumatoid arthritis receiving a standard dose of oral weekly methotrexate: association with significant decreases in creatinine clearance and renal clearance of the drug after 6 months of therapy. J Rheumatol. 1995;22(1):38–40.
- Seideman P, Müller-Suur R, Ekman E. Renal effects of low dose methotrexate in rheumatoid arthritis. J Rheumatol. 1993;20(7):1126–1128.
- Sparks JA, Vanni KMM, Sparks MA, et al. Effect of low-dose methotrexate on eGFR and kidney adverse events: a randomized clinical trial. J Am Soc Nephrol. 2021;32(12):3197–3207.
- Lee JS, Oh JS, Kim Y-G, et al. Methotrexate-related toxicity in patients with rheumatoid arthritis and renal dysfunction. Rheumatol Int. 2020;40(5):765–770.
- Sumida K, Molnar MZ, Potukuchi PK, et al. Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int. 2018;93(5):1207–1216.
- Zee J, Mansfield S, Mariani LH, et al. Using all longitudinal data to define time to specified percentages of estimated GFR decline: a simulation study. Am J Kidney Dis. 2019;73(1):82–89.
- Dobry AS, Quesenberry CP, Ray GT, et al. Serious infections among a large cohort of subjects with systemically treated psoriasis. J Am Acad Dermatol. 2017;77(5):838–844.
- Dávila-Seijo P, Dauden E, Descalzo MA, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol. 2017;137(2):313–321.
- Garcia-Doval I, Cohen AD, Cazzaniga S, et al. Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti–tumor necrosis factor agents versus classic therapies: prospective meta-analysis of psonet registries. J Am Acad Dermatol. 2017;76(2):299–308.e16.
- Wang Y-C, Lin Y-H, Ma S-H, et al. Infection risk in psoriatic patients receiving tumour necrosis factor inhibitors: a 20-year systematic review and meta-analysis of randomized controlled trials. J Eur Acad Dermatol Venereol. 2022;36(12):2301–2315.
- Prey S, Paul C. Effect of folic or folinic acid supplementation on methotrexate-associated safety and efficacy in inflammatory disease: a systematic review. Br J Dermatol. 2009;160(3):622–628.

