Abstract
Study purpose: New treatments for atopic dermatitis (AD) are emerging; however, little is known about the treatment preferences of patients with mild-to-moderate AD. To measure patients’ preferences, a cross-sectional, web-based discrete choice experiment (DCE) survey was developed and administered to 300 adults in the United States with a self-reported physician diagnosis of mild-to-moderate AD.
Materials and methods: In the DCE, respondents evaluated pairs of hypothetical AD treatment profiles defined by efficacy, risk, and mode and frequency of administration attributes. The DCE data were analyzed using a random parameters logit model. Subgroup analysis was used to investigate preference heterogeneity.
Results: The results revealed achieving clear or almost clear skin within 3-4 months of treatment was the most important attribute relative to all other study attributes. The results indicated that a topical cream applied twice daily was preferred to systemic treatments. Subgroup analysis revealed that respondents with lower self-assessed disease burden were more likely to choose topical over systemic treatments and less averse to the risk of pain, burning, and/or stinging from the medicine (all other treatment features remaining equal) than respondents with higher self-assessed disease burden.
Conclusion: The results of this study can help inform shared decision-making to manage mild-to-moderate AD.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory disease that is associated with a substantial disease burden. It can have significant lifelong impacts on dimensions of quality of life including social interactions, emotional and behavioural changes, ability to perform physical activities, and productivity at work or school; it can also increase risks of other comorbidities [Citation1]. The onset of AD commonly occurs during early childhood. While approximately 60% of children diagnosed with AD will experience remission by adolescence, 40% will have a disease that persists into adulthood [Citation2]. Signs of AD can vary but typically include dry, red, and inflamed skin patches. The hallmark symptom of AD is pruritus, which may be extremely intense and disruptive to daily life.
Many treatment guidelines for AD in the United States (US) and Europe employ a stepwise approach that depends largely on the severity of the patient’s disease. For over 60 years, topical corticosteroids (TCSs) have been the treatment mainstay for acute inflammation caused by AD, followed by the use of topical calcineurin inhibitors as second-line therapy for patients who become refractory to TCSs or when TCSs are not recommended. However, the use of a TCS is not recommended long-term due to safety/tolerability concerns [Citation3]. Furthermore, many patients with mild-to-moderate disease fail to achieve long-term symptom control with TCSs and often relapse and are on the cusp between remaining on topicals or seeking systemic alternatives. This illustrates ongoing unmet needs in the treatment of mild-to-moderate AD.
There are several newer steroid-free therapies approved by the US Food and Drug Administration (including dupilumab, tralokinumab, crisaborole cream, ruxolitinib cream, upadacitinib, and abrocitinib), with more under development. These therapies vary in terms of treatment benefits, side effects, and modes of administration. As the AD treatment landscape continues to evolve, it is important to understand how patients with mild-to-moderate AD that is not well-controlled on a TCS evaluate differences among treatment benefits and risks as well as among mode and frequency of administration.
To date, there are only 2 published studies that quantified patient preferences regarding attributes of AD treatments. A Japanese study focused on the attributes of injectable AD treatments among patients with moderate to very severe AD [Citation4]. The other study focused on attributes of systemic treatments among patients with moderate-to-severe AD in the US and United Kingdom [Citation5]. None addressed patient preferences regarding the treatment of mild-to-moderate AD. We hypothesized that the preferences of patients with mild-to-moderate AD differ from those previously observed with moderate-to-severe disease.
The primary objective of this study was to use a discrete-choice experiment (DCE) to (1) quantify patients’ preferences for attributes associated with AD treatments for mild-to-moderate AD, (2) estimate the relative importance of treatment attributes, and (3) explore heterogeneity in preferences between subgroups of patients.
Materials and methods
Study design
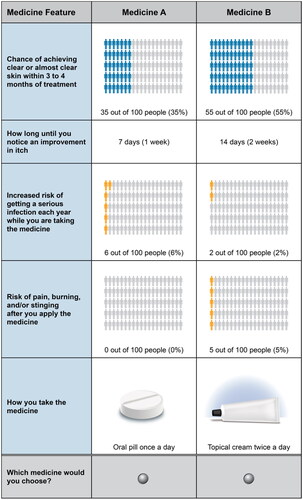
Development, administration, and analysis of the DCE survey followed good research practice guidelines [Citation6–8] and Center for Devices and Radiological Health guidelines [Citation9]. The survey instrument asked respondents to assume their doctor told them about new medicines for AD that may be able to control their symptoms better than the currently prescribed medicine. Respondents were then presented with a series of choices between 2 hypothetical treatment profiles for AD (Medicine A or Medicine B). The hypothetical AD treatment profiles varied in 5 attributes: treatment benefits (chance of clear or almost clear skin within 3 to 4 months of treatment; time until onset of action), treatment risks (increased risk of serious infection; risk of pain, burning, and/or stinging after application), and treatment administration (topical cream twice a day, oral pill or tablet once a day, and self-injection under the skin once every other week) (). The attributes were selected based on endpoints from clinical trials associated with approved and investigational therapies for mild-to-moderate AD [Citation10–12], published data related to the treatment of AD, and prescribing information for currently available AD treatments [Citation13–14]. The levels were selected to be meaningful and salient to respondents and to encompass the potential range of efficacy improvements or risk increases seen in clinical trials or clinical practice.
Table 1. Attributes and levels included in the discrete-choice experiment.
The hypothetical treatment profiles were generated by an experimental design [Citation15–16]. This design produced 72 unique choice questions, each of which was assigned to 1 of 6 blocks of 12 choice questions. Each respondent was randomly assigned to 1 of the 6 blocks from the experimental design. Finally, to avoid potential bias due to learning and fatigue, the order of the choice questions in each block was randomized for each respondent. A sample choice question is presented in .
Before the survey was administered, the survey instrument was pretested using web-based interviews, each lasting approximately 60 min, with 15 eligible participants with self-reported mild-to-moderate AD. The pretest interviews assessed whether the survey questions were comprehensible and whether the attributes and levels were comprehensive, relevant, and appropriately described. Based on initial pretest findings, the survey was revised to incorporate minor changes to clarify the question text and the descriptions of the attributes. The revisions were then tested in subsequent interviews until the pretests confirmed that the survey was performing as intended. The final survey instrument was administered by Global Perspectives, a health-related market research firm, to members of their online panel as well as through their partner panels from July through September 2021.
Study population
Eligible respondents were aged ≥ 18 years, were living in the US, and were able to read and speak English in order to provide informed consent. Eligible respondents (1) had a self-reported physician diagnosis of AD, (2) had a self-assessed body surface area (BSA) of 20% or less covered with AD lesions, (3) were currently taking prescription medicine to treat their AD, and (4) self-reported that their current treatment did not adequately control their AD (i.e. either somewhat or strongly disagreed with the statement ‘I feel my current treatments are working to keep my eczema under control’). The sample was recruited using online panels. All respondents provided electronic informed consent. The survey was granted an exemption from review by RTI International’s institutional review board (Federal Wide Assurance #3331).
Statistical analysis
Responses to the DCE questions were analyzed using a random-parameters logit (RPL) model, which relates respondents’ treatment choices to the attribute levels of each treatment profile in the choice questions. An RPL model produces a relative preference weight for each attribute level. The preference weight estimates themselves do not have an intuitive interpretation as a stand-alone estimate. Rather, differences in preference weights corresponding to 2 levels of the same attribute are used to describe the relative importance of changes within an attribute. The RPL model accounts for unobserved preference heterogeneity among respondents by estimating a distribution around each mean preference parameter, and it also accounts for the fact that each respondent made multiple treatment choices over a series (panel) of choice questions [Citation17–19].
Statistical analysis of the DCE data was conducted following good research practice guidelines published by the International Society for Pharmacoeconomics and Outcomes Research [Citation8]. The analyses were performed with STATA 16.0 (College Station, TX). Unless specified otherwise, an α-level of 0.05 (or 5%) was used to evaluate statistical significance. All attribute levels in this study were effects coded such that the mean effect of each attribute was normalized at zero. A one-sample t test was used to determine the statistical significance of differences between adjacent attribute levels.
Preference weights were used to estimate the conditional relative importance – the maximum change in utility achievable with any attribute, conditional on the levels of the attributes selected in the study design. Conditional relative importance was calculated as the difference between the preference weights for the most and least preferred attribute levels. These differences are summed across attributes, and the sum is scaled to 100. The conditional importance of each attribute is a percentage of this total.
Preference weights were also used to estimate the maximum acceptable risks (MARs), or the level of risk that offsets exactly the increase in utility attributable to an improvement in the levels of other treatment attributes. Estimates of MARs were calculated for getting a serious infection or for experiencing pain, burning, and/or stinging in exchange for percentage improvements in the chance of achieving clear or almost clear skin in 3 to 4 months, numeric reductions in how long until a noticeable improvement in itch, and changes in the mode of administration. These calculations hold constant the levels of all attributes other than the 1 attribute that is changing.
Subgroup analyses were used to determine whether average preferences varied systematically among mutually exclusive subgroups of respondents with (1) different levels of self-assessed disease burden and (2) AD lesions in different locations of the body. Supplemental Appendix A describes these analyses in detail.
Results
Respondent characteristics
The final sample included 300 respondents. Respondents had a mean age of 39.9 years (standard deviation, 13.2 years); 65% identified as female. Most (63.3%) had at least a 4-year college degree and identified as White (69.7%) (). Most respondents had a BSA of less than 10% covered with AD lesions (71.7%) and had taken a prescription topical treatment (91.3%); among those who had ever taken a prescription topical treatment, most were still taking a prescription topical treatment at the time of the survey (93.1%).
Table 2. Characteristics of the respondents (N = 300).
Preference weights and conditional relative attribute importance
shows mean preference weight estimates for each attribute level. With one exception, all adjacent levels of the same attribute were statistically significantly different from one another (p ≤ 0.05), indicating that respondents differentiated between the levels when making treatment choices in the survey. The exception was that respondents did not differentiate between 1 day or less or 7 days until they would notice an improvement in itch. The lack of a statistically significant difference in preference weights between 2 levels of an attribute may mean that respondents were indifferent between those levels; that is, the change from one level to another did not have a statistically significant impact on treatment choice.
Figure 2. Attribute preference weights (N = 300). DCE = discrete-choice experiment. Attributes are presented in the order in which they appeared in the DCE questions. The change in utility associated with the difference between 2 levels of a given attribute is represented by the difference between the preference weights for those attribute levels. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility or having greater relative importance in treatment choice. The vertical bars around each mean preference weight represent the 95% confidence interval around the point estimate.
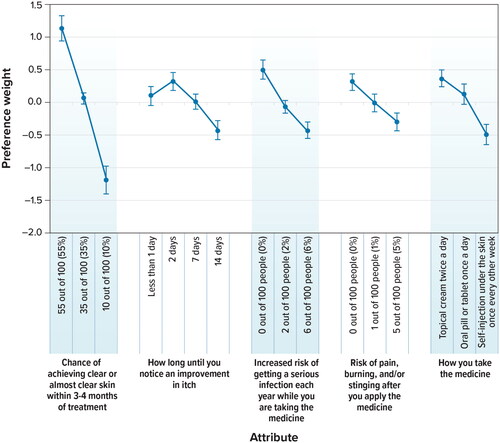
shows that the relative importance of a 45 percentage point improvement (from 10% to 55%) in the chance of clear or almost clear skin within 3 to 4 months of treatment was 2.31. The relative importance of lowering the treatment-related risk of serious infection from 2% to 0% was 0.57. Therefore, improving the chance of achieving clear or almost clear skin within 3 to 4 months of treatment from 10% to 55% was approximately 4 times (2.31 ÷ 0.57 = 4.05) as important as reducing the risk of getting a serious infection from 2% to 0%.
The results also indicated that a topical cream applied twice daily was preferred to a once-daily oral pill (p = 0.05) and a self-injection under the skin administered once every other week (p < 0.001). Furthermore, using a topical cream versus an injectable treatment was 1.5 times more important to respondents than reducing the risk of getting a serious infection from 2% to 0%; 1.4 times more important than reducing the risk of pain, burning, and/or stinging from 5% to 0%; and about as important as reducing the time until onset of action from 14 days to 2 days.
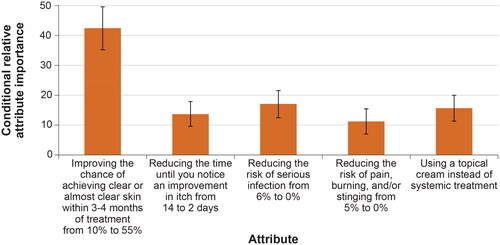
shows the conditional relative attribute importance of changing each attribute from the least preferred level to the most-preferred level, or the maximum change in utility achievable for each attribute, which indicates the importance of the attributes relative to one another. Over the ranges presented in the survey, respondents placed the greatest importance on the change in the chance of clear or almost clear skin within 3 to 4 months of treatment from 10% to 55%. This was followed by, in decreasing order of importance, the reduction in the risk of serious infection from 6% to 0%; the use of a topical treatment versus a systemic treatment; a reduction in the time until an improvement in itch from 14 days to 2 days; and a reduction in the risk of pain, burning, and/or stinging from 5% to 0% after applying the medicine. However, the importance of a 45 percentage point increase in the chance of having clear or almost clear skin within 3 to 4 months of treatment was estimated to be statistically significantly different and higher than the changes in other attributes presented in the survey, while the importance of changes in the other attributes was relatively similar.
Figure 3. Conditional relative attribute importance (N = 300). DCE: discrete-choice experiment. Note: The conditional relative importance is the difference between the preference weights on the most influential attribute level and the least influential attribute level. These differences are summed across attributes, and the sum is scaled to 100. The conditional importance of each attribute is a percentage of this total. The vertical bars surrounding each relative importance weight estimate denote the 95% confidence interval (computed by the delta method). Attributes are presented in the order in which they appeared in the DCE questions.
Maximum acceptable risk
Maximum acceptable risks present an alternative way to quantify the relative importance of changes from one level of an attribute to another: the average additional increase in risk that respondents would accept for a given change or improvement in the levels of other treatment attributes. shows that, on average, respondents were willing to accept more than 6% additional risk of serious infection and more than 5% additional risk of pain, burning, and/or stinging, which are above the highest level of risks presented in the survey, for all levels of improvement in the chance of achieving clear or almost clear skin within 3 to 4 months of treatment. Furthermore, for a change in the mode of administration from a self-injection under the skin once every other week to a topical cream twice a day, respondents were willing to accept, on average, a 5% additional risk of getting a serious infection each year while taking the medicine and a greater than 5% additional risk of pain, burning, and/or stinging after applying for the medicine. Respondents were also willing to accept, on average, a 1.8% additional risk of serious infection and a 3.9% additional risk of pain, burning, and/or stinging in order to reduce the number of days until noticing an improvement in itch from 14 days to less than 1 day.
Table 3. Maximum acceptable risk calculations (N = 300).
Subgroup analyses
We tested for differences in preferences by self-reported disease burden and whether respondents had lesions in sensitive areas (see Supplemental Appendix A). Of these 2 subgroups, only the differences between respondents with low versus high self-assessed disease burden were statistically significant (p < 0.001).
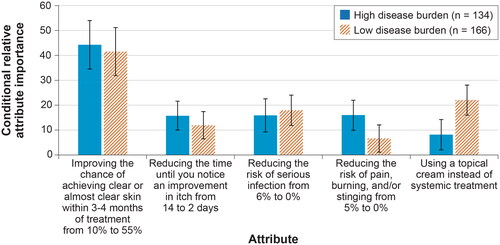
Respondents with lower self-assessed disease burden (equal to or lower than the median value of 6) (n = 166) and respondents with high self-assessed disease burden (greater than the median value of 6) (n = 134) both placed the most importance on improving the chance of achieving clear or almost clear skin within 3 to 4 months of treatment from 10% to 55% (). There were also statistically significant differences in conditional relative attribute importance weights across the 2 groups for a mode of administration. Respondents with low self-assessed disease burden placed more importance on and thus experienced a greater change in the utility from the mode of administration –specifically, changing to a topical cream from a self-injection than respondents with high self-assessed disease burden. Respondents with a high self-assessed disease burden placed greater relative importance on the risk of pain, burning, and/or stinging than the respondents with a low self-assessed disease burden, but the difference in conditional relative attribute importance was not statistically significant ().
Figure 4. Attribute preference weights: disease burden subgroups (N = 300). DCE: discrete-choice experiment. Note: Attributes are presented in the order in which they appeared in the DCE questions. The change in utility associated with the difference between 2 levels of a given attribute is represented by the difference between the preference weights for those attribute levels. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility or having greater relative importance in treatment choice. The vertical bars around each mean preference weight represent the 95% confidence interval around the point estimate.
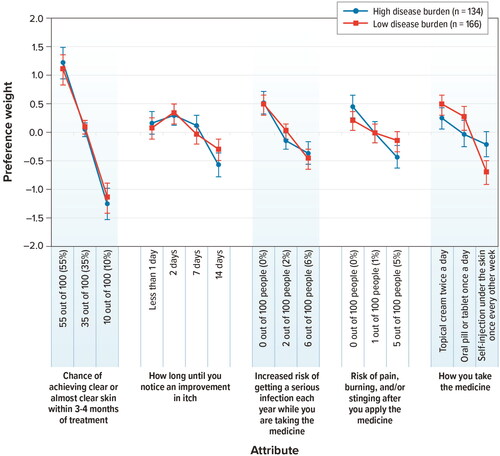
Figure 5. Conditional relative attribute importance: disease burden subgroup (N = 300). DCE: discrete-choice experiment. The conditional relative importance is the difference between the preference weights on the most influential attribute level and the least influential attribute level. These differences are summed across attributes, and the sum is scaled to 100. The conditional importance of each attribute is a percentage of this total. The vertical bars surrounding each relative importance weight estimate denote the 95% confidence interval (computed by the delta method). Attributes are presented in the order in which they appeared in the DCE questions.
Respondents with low self-assessed disease burden, on average, were willing to –cept a greater than 6% additional risk of serious infection and a greater than 5% additional risk of pain, burning, and/or stinging for a change in the mode of administration from a self-injection twice a week to a topical cream twice a day (Table A1, Supplemental Appendix A). Respondents with high self-assessed disease burden also demonstrated a willingness to accept risk for changes in mode; however, they were only willing to accept an approximate 1.3% additional risk of serious infection and an approximate 1% additional risk of pain, burning, and/or stinging for the same change (self-injection to topical cream).
Respondents with both high and low self-assessed disease burden were willing to accept a greater than 6% additional risk of serious infection and a greater than 5% additional risk of pain, burning, and/or stinging – which are above the highest level of risks presented in the survey– for all changes in the chance of achieving clear or almost clear skin within 3 to 4 months of treatment (i.e. from 10% to 35%, from 35% to 55%, and from 10% to 55%).
Discussion
This DCE study explored the importance of differences in treatment benefits (chance of achieving clear or almost clear skin within 3 to 4 months of treatment, and time until a noticeable improvement in itch) relative to differences in other treatment characteristics (such as increased risk of getting a serious infection; increased risk of pain, burning, and/or stinging after applying the medicine; and change in mode of administration) among patients with mild-to-moderate AD in the US. Given the range of attributes and levels included in the survey, changes in the chance of achieving clear or almost clear skin within 3 to 4 months of treatment were most important to respondents. Our results show that for patients with mild-to-moderate AD, changes in the chance of achieving clear or almost clear skin within 3 to 4 months and changing the mode of administration from a self-injection to a topical cream yielded greater changes in utility than reducing the risk of treatment-related serious infection from 2% to 0%. On average, respondents would tolerate the greatest increases in the risks of serious infection and pain, burning, and/or stinging for increases in the chance of achieving clear or almost clear skin in 3 to 4 months of treatment (greater than 6% and 5%, respectively) and for use of topical rather than self-injection treatments (5.15% and >5%, respectively).
Subgroup analysis revealed that respondents with low self-assessed disease burden had different preferences than respondents with high self-assessed disease burden, largely driven by the mode of administration and the risk of pain, burning, and/or stinging after applying the treatment. In particular, compared with respondents with a high self-assessed disease burden, those with a low self-assessed disease burden showed a stronger preference for topical administration over self-administered injection. The MAR results across the subgroups confirmed these findings, as respondents with high self-assessed disease burden were willing to accept much lower risks for changes in the mode of administration. These differences could be due to an unwillingness to commit to a systemic therapy if the perceived disease burden is low or to the difficulty in adhering to a topical regimen among patients with lesions covering a higher percentage of their BSA (i.e. BSA > 10%). For instance, in the low disease burden subgroup, approximately 80% of patients self-reported a BSA of less than 10%, while approximately 60% of patients in the high disease burden subgroup self-reported the same BSA level.
This study was only the second to quantitatively estimate preferences for AD treatments in the US. To our knowledge, it was the first to assess preferences among a broader set of AD treatment attributes that include a topical application as a potential mode of administration as well as topical mode–related side effects (such as the risk of pain, burning, and/or stinging) in a population with milder disease. Boeri et al. [Citation5] conducted a DCE study of patients with moderate-to-severe AD in the US and United Kingdom to quantify the preferences for attributes of systemic treatments for AD. Similar to the present study, Boeri et al. found that for efficacy, improving the chance of clear or almost clear skin within 16 weeks was most important. However, the avoidance of an annual risk of malignancy was the most important attribute relative to the other attributes included in that study. They also found that respondents preferred an oral pill once daily to an injection every 2 weeks; however, they did not include a topical application as an option, which was likely due to the disease severity of the sampled population. In contrast to this study, Boeri et al. [Citation5] found that improving the chance of clear or almost clear skin within 16 weeks of treatment was almost as important as reducing the time until the onset of itch relief among patients with moderate-to-severe AD. In the present study, improving the chance of clear or almost clear skin within 16 weeks of treatment was more than 3 times as important as reducing the time until onset of itch relief from 2 weeks to 2 days.
Okubo et al. [Citation4] conducted a DCE study of patients with moderate to very severe AD and physicians who treat patients with AD in Japan to quantify the preferences for attributes of injectable treatments for AD. The results of the DCE revealed that patients cared the most about the risk of mild-to-moderate side effects, time until onset of action, and efficacy associated with a reduction in itch. However, these results were different for respondents with moderate AD than they were for respondents with severe or very severe AD. Respondents with moderate AD cared more about reducing the risk of mild-to-moderate side effects than did respondents with severe or very severe AD. In contrast, respondents with severe or very severe AD placed more importance on a percentage reduction in itching. In the present study, respondents with higher self-assessed disease burden placed more importance on reducing the time until onset of itch than did respondents with lower self-assessed disease burden, although this difference was not statistically significant.
The results of this study should be interpreted in the context of limitations related to the survey instrument and sample. First, the scope of this study was limited to the preferences of adult patients; therefore, the preferences of pediatric and/or adolescent patients were outside the scope of this study. It would be important for future studies to further explore preferences among these populations. Second, a potential limitation of this study (and all voluntary studies) is selection bias resulting in non-representativeness, which may lead to an underestimate or overestimate of respondents’ preferences. The final survey was administered online. Research has shown that results from online stated-preference surveys are, in general, not statistically significantly different from those elicited through face-to-face interviews [Citation20–21]. However, the online setting of the survey also may have influenced respondents’ choices. In addition, respondents in this study evaluated hypothetical treatments, and their choices among these treatments do not have the same significance as choices involving actual treatment decisions. Actual treatment choices may depend on a number of contextual factors that were beyond the scope of this study as well as the potential for treatment adherence issues in the real world setting.
In conclusion, our results indicate that among respondents with mild-to-moderate AD, an improvement in the chance of clear or almost clear skin within 3 to 4 months of treatment was the most important driver of treatment choices. Respondents generally preferred using a topical cream to a systemic treatment, and this preference was more pronounced among respondents with lower self-assessed disease burden than among respondents with higher self-assessed disease burden. As new treatments for AD become available, patients’ preferences for different treatment benefits and risks, as well as how patients evaluate mode and frequency of administration, can help better inform shared decision-making when selecting therapies to manage mild-to-moderate AD.
ijdt_a_2215356_sm3025.pdf
Download PDF (207.7 KB)Acknowledgements
The authors thank the individuals who participated in the study. Kimberly Moon of RTI Health Solutions provided overall project management for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Access to individual patient-level data is not available for this study. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960
Additional information
Funding
References
- Eichenfield LF, Stein Gold LF. The disease burden of atopic dermatitis. Semin Cutan Med Surg. 2017;36(4S):1–10.
- Bylund S, Kobyletzki LB, Svalstedt M, et al. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160.
- Eichenfield LF, Wynnis TL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351.
- Okubo Y, Ho KA, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–830.
- Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatolog Treat. 2022; 33(3):1449–1458.
- Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health –a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis discrete-choice experiment experimental design good research practices task force. Value Health. 2013;16(1):3–13.
- Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis experimental design task force. Value Health. 2016;19(4):300–315.
- Food and Drug Administration [Internet]. Silver Spring (MD): U.S. Department of Health and Human Services Food and Drug Administration; 2016. Patient preference information – voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling - guidance for industry, Food and Drug Administration staff, and other stakeholders; 2016 Aug 24 [cited 13 Mar 2023]. Available from: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM446680.pdf.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020; 156(8):863–873.
- Paller AS, Wynnis LT, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503.e6.
- Simpson EL, Bieber T, Guttman-Yassky LA, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- Pfizer. 2016. Highlights of prescribing information: Crisaborole. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/207695s007s009s010lbl.pdf.
- Regeneron/Sanofi. 2017. Highlights of prescribing information: dupilumab. Available from: https://www.regeneron.com/downloads/dupixent_fpi.pdf.
- Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31(4):545–557.
- Kuhfeld W. Marketing research methods in SAS: experimental design, choice, conjoint, and graphical techniques. Cary, NC: SAS Institute Inc.; 2010.
- McFadden D, Train K. Mixed MNL models for discrete response. J Appl Econ. 2000;15(5):447–470.
- Train K. Discrete choice methods with simulation. 2nd ed. Cambridge, MA: cambridge University Press; 2009.
- Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Alberini A, editors. Applications of simulation methods in environmental and resource economics. Dordrecht, Netherlands: Springer; 2005.
- Nielsen JS. Use of the internet for willingness-to-pay survey: a comparison of face‑to‑face. And Web-Based Interviews. Res Energy Econ. 2011;33(1):119–129.
- Marta-Pedroso C, Freitas H, Domingos T. Testing for the survey mode effect on contingent valuation data quality: a case study of web based versus in-person interviews. Ecol Econ. 2007;62(3-4):388–398.