Abstract
Background
Baricitinib is an oral selective Janus kinase 1/2 inhibitor approved for moderate-to-severe atopic dermatitis (AD) in adults.
Objectives
To evaluate absolute Eczema Area and Severity Index (EASI) and SCORing of Atopic Dermatitis (SCORAD) outcomes over 16 weeks and to link disease severity categories to quality of life (QoL) improvements.
Methods
This post-hoc analysis included patients enrolled in Phase3 monotherapy (BREEZE-AD1/AD2) and topical corticosteroid (TCS) combination therapy (BREEZE-AD7) trials and analyzed baricitinib 2 and 4 mg vs. placebo. Categorical outcomes were analyzed using Fisher’s exact test.
Results
Significantly more baricitinib-treated patients reached EASI ≤ 7 and SCORAD < 25 as early as week 1 in monotherapy and week 2 in TCS combination therapy, compared to placebo. Significant response vs. placebo was sustained until week 16 for EASI ≤ 7 (AD1/2 [p-value vs. placebo]: 2 mg = 19.9%, 4 mg = 25.4% [p = 0.001] and AD7: 2 mg = 40.4% [p = 0.087], 4 mg = 48.6% [p = 0.003]) and SCORAD < 25 (AD1/2: 2 mg = 12.2%, 4 mg = 19.4% [p = 0.001] and AD7: 2 mg = 30.3% [p = 0.025], 4 mg = 34.2% [p = 0.004]) severity categories. These effects were accompanied by rapid improvements in QoL.
Conclusion
Baricitinib-treated patients rapidly achieved recommended absolute EASI and SCORAD treatment outcomes which were sustained until week 16. Improvements in QoL were greater than EASI severity categories reflected, indicating that physician-assessed scores do not necessarily correlate with patients’ impression of AD severity.
Introduction
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease characterized by eczematous lesions and periods of acute worsening with flares (Citation1–3). The effects of AD extend beyond cutaneous signs as patients experience intense pruritus, sleep disturbances, and skin pain that ultimately reduce their quality of life (QoL). Accordingly, AD has been ranked as the leading cause of non-fatal health burden in skin conditions (Citation4,Citation5).
In the last decade, progress has been made in standardizing the assessment of AD in randomized clinical trials (RCT) and clinical practice. A recent report by the global Harmonizing Outcome Measures in Eczema initiative recommends the Eczema Area and Severity Index (EASI) as the preferred instrument to objectively assess clinical signs of AD (Citation6). This tool is widely used in RCTs and assesses the severity of AD lesions, but does not perform well in low disease severity (Citation7). Also taking into account the importance of subjective symptoms in defining AD severity, the European Task Force on AD (ETFAD) recommended using the SCORing of Atopic Dermatitis (SCORAD), a composite score including patient-reported itch intensity, sleep disturbances, and physician-reported AD severity (Citation8).
In RCTs, relative response outcomes, including the percentage of patients having reached ≥75% improvement from baseline in EASI or SCORAD, are usually used to determine AD severity over time. However, relative outcomes might have limitations as they depend on disease severity at baseline, which can be unknown in clinical practice, particularly for patients switching to another treatment option. Furthermore, as patients with severe disease can achieve relative improvements from baseline despite having residual skin lesions, relative improvements might not inform on actual disease severity at the time of analysis. Therefore, absolute disease severity can provide important information.
Accordingly, the consensus-based European guidelines for the treatment of AD recommend defining AD severity using absolute SCORAD categories of mild (<25), moderate (25–50), and severe (>50) (Citation9). Similar severity categories were defined for EASI: clear/almost clear or mild (0.0–7.0), moderate (7.1–21.0), and severe (21.1–72.0) (Citation10). Additionally, minimal clinically important differences (MCID) of ≥6.6 and ≥8.7-point improvements from baseline were defined for EASI and SCORAD, respectively, to determine if changes in absolute scores reflect a real change in disease severity (Citation11).
Both relative and absolute EASI and SCORAD scores, along with patient-assessed outcomes, were recommended treatment goals in a recent international treat-to-target consensus guiding systemic treatment decisions in adults with moderate-to-severe AD (Citation12). Specifically, achieving absolute treatment targets of EASI ≤ 7 and SCORAD < 25, or relative EASI 75 and SCORAD 75, were recommended six-month treatment goals in the consensus guide in addition to an absolute patient global assessment (PGA) ≤ 2. Disease domains evaluating itch or QoL were proposed alternatives to EASI and SCORAD (Citation12). These recommendations, along with others (Citation13), highlight the importance of considering not only reductions in AD lesions but also other treatable traits. To assess the full impact of the disease and to evaluate AD severity, both physician-reported and patient-reported outcome measures should be taken into account.
Baricitinib (BARI), an oral selective inhibitor of Janus kinase 1 and 2, is approved in the European Union, Japan, and several other countries for treating adults with moderate-to-severe AD who are candidates for systemic therapy. BARI demonstrated fast and maintained efficacy on the clinical signs and symptoms of moderate-to-severe AD in adults enrolled in randomized, placebo (PBO)-controlled Phase 3 trials in both monotherapy (BREEZE-AD1, BREEZE-AD2) and topical corticosteroid (TCS) combination therapy (BREEZE-AD7) for 16 weeks, with data available up to 68 weeks from the long-term extension study (BREEZE-AD3) (Citation14–16).
The objectives of this post-hoc analysis were to evaluate absolute EASI and SCORAD outcomes in patients treated with BARI monotherapy or TCS combination therapy by assessing absolute changes from baseline, absolute treatment targets, and MCIDs over the 16-week treatment period. Additionally, we aimed to analyze the relationship between QoL improvements and objective disease severity categories to help make informed decisions in clinical practice using a holistic approach.
Materials and methods
Study design and participants
This post-hoc analysis included data from patients enrolled in the monotherapy trials, BREEZE-AD1 (NCT03334396) (n = 497) and BREEZE-AD2 (NCT03334422) (n = 490), and the TCS combination therapy trial, BREEZE-AD7 (NCT03733301) (n = 329). Detailed study designs have been previously published (Citation14,Citation15). Briefly, BREEZE-AD1, BREEZE-AD2, and BREEZE-AD7 were independent, 16-week randomized, double-blind, parallel-group, PBO-controlled trials. Patients were randomized 2:1:1:1 to receive once-daily PBO, BARI 1, 2, or 4 mg in BREEZE-AD1 and BREEZE-AD2, and 1:1:1 to receive once-daily PBO, BARI 2 or 4 mg in BREEZE-AD7. Enrolled patients were ≥18 years old, had an AD diagnosis for ≥12 months before screening, and had a history of inadequate response to topical treatments ≤6 months before screening. Patients had moderate-to-severe AD defined as an EASI score ≥16, a Validated Investigator’s Global Assessment of AD (vIGA-AD) score ≥3, and ≥10% body surface area (BSA) at screening and baseline.
All patients discontinued topical and systemic therapies 2 and 4 weeks before randomization, respectively. Topical therapies were not allowed in BREEZE-AD1 and BREEZE-AD2 except as a rescue therapy. Background moderate- and/or low-potency TCS use was allowed in BREEZE-AD7 for active lesions. This analysis pooled data from BREEZE-AD1 and BREEZE-AD2, while BREEZE-AD7 was analyzed separately, and evaluated patients treated with either PBO, BARI 2 or 4 mg, which were tested within the primary endpoints of the studies, through W16 (Citation14,Citation15,Citation17). All patients provided written informed consent before enrollment. All studies were conducted with the approval of each center’s institutional review board or independent ethics committee and in accordance with the guiding principles of the Declaration of Helsinki.
Efficacy endpoints
EASI was used as skin signs only score and SCORAD as a composite score assessing skin signs and subjective symptoms. Efficacy endpoints in this post-hoc analysis included the mean change from baseline in absolute EASI and SCORAD scores and the proportion of patients achieving EASI ≤ 7, SCORAD < 25, or an MCID in EASI or SCORAD through W16. To investigate the clinical meaningfulness of MCIDs, the proportions of patients reaching MCIDs in EASI and SCORAD were categorized by predefined EASI and SCORAD severity categories, respectively, at W16 (Citation9,Citation10). To investigate the relationship between improvements in skin signs and QoL, the proportion of patients with a baseline Dermatology Life Quality Index (DLQI) > 5 achieving a DLQI ≤ 5 (indicates no/minimal impact of AD on QoL) was stratified by EASI disease category and assessed at W1, W4, and W16 (Citation18).
Statistical analysis
Statistical software SAS version 7.1 was used in this analysis. Continuous outcomes were compared using mixed models repeated measures (MMRM) analysis with fixed effects for treatment, region, baseline severity, visit, treatment-by-visit interaction, and baseline value-by-visit interaction. Categorical outcomes were compared using the Fisher’s exact test. Missing data (patients receiving rescue therapy or discontinuing treatment) were imputed using non-responder imputation for categorical variables. An MMRM analysis was performed for missing data for continuous variables. Two-sided statistical tests were performed where p < 0.05 was considered statistically significant. Due to the exploratory nature of these analyses, no correction for multiplicity was performed.
Table 1. Demographics and baseline disease characteristics.
Results
Patient baseline demographics and AD disease characteristics
In total, 1,316 patients were included in our analysis. Patients had severe AD based on mean scores for EASI (≥32.3 across treatment arms for BREEZE-AD1 and -AD2, and ≥28.5 for BREEZE-AD7) and SCORAD (≥67.9 across treatment arms for BREEZE-AD1 and -AD2, and ≥66.6 for BREEZE-AD7), reflecting severe disease as per predefined disease severity categories (21.1–72.0 for EASI and >50 for SCORAD) (Citation9,Citation10). A high proportion of patients demonstrated severe AD with a vIGA-AD = 4 (≥44.4% across treatment arms) and widespread BSA involvement (≥48.1% across treatment arms). Severe AD had a substantial negative impact on patients’ QoL as ≥83.7% and ≥84.4 of patients across treatment arms for BREEZE-AD1 and -AD2 and BREEZE-AD7, respectively, had a DLQI > 5 (at least moderate effect on QoL) ().
Reduction in absolute EASI score over 16 weeks
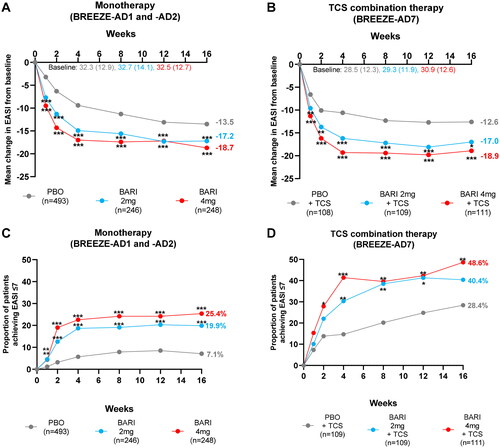
From as early as W1, patients treated with BARI 2 mg and 4 mg monotherapy and TCS combination therapy had significantly greater reductions in absolute EASI score from baseline, compared with patients treated with PBO. At Week 4, EASI scores decreased by −9.4, −14.9, and −17.0 in monotherapy and by −10.6, −16.2, and −19.3 in TCS combination therapy for patients treated with PBO, BARI 2 and 4 mg, respectively, representing decrease in mean EASI from severe to moderate severity category. This reduction was sustained through to W16, where absolute mean EASI was reduced to 15.5 for BARI 2 mg and to 13.8 for BARI 4 mg in monotherapy and to 12.3 for BARI 2 mg and to 12.0 for BARI 4 mg in TCS combination therapy, continuing in mean moderate EASI range ().
Importantly, these rapid reductions in absolute EASI scores translated into significantly more patients treated with BARI 2 or 4 mg monotherapy (BREEZE-AD1 and BREEZE-AD2) achieving EASI ≤ 7 vs. those treated with PBO at all timepoints from W1 through W16 (). In the TCS combination therapy study (BREEZE-AD7), significantly more patients treated with BARI 4 mg achieved EASI ≤ 7 vs. PBO at all timepoints from W2 through W16, and almost half (48.6%) of BARI 4 mg-treated patients achieved EASI ≤ 7 by W16 ().
Figure 1. Reduction in absolute EASI scores over 16 weeks in patients treated with BARI monotherapy or TCS combination therapy. Least square mean change from baseline to W16 in absolute EASI scores in patients from (A) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (B) the TCS combination therapy study (BREEZE-AD7). The proportion of patients achieving EASI ≤ 7 through W16 from (C) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (D) the TCS combination therapy study (BREEZE-AD7). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. PBO. BARI: baricitinib; EASI: Eczema Area and Severity Index; PBO: placebo; TCS: topical corticosteroid.
Reduction in absolute SCORAD score over 16 weeks
Significantly greater reductions in absolute SCORAD score from baseline in patients treated with BARI monotherapy or TCS combination therapy vs. PBO were seen at all timepoints through W16. The reduction in absolute SCORAD score had a similar time course to the reduction in absolute EASI score, now combining measures of both signs and symptoms of AD. Absolute SCORAD score decreased rapidly by W1 and continued to decrease until W4, after which, the reduction was maintained through W16. At Week 4, SCORAD scores decreased by −9.9, −21.9, and −26.3 in monotherapy and by −16.8, −29.1, and −35.0 in TCS combination therapy for patients treated with PBO, BARI 2 and 4 mg, respectively, representing a decrease from mean SCORAD in severe to moderate category. This reduction was sustained through to W16, where absolute mean SCORAD was reduced to 44.3 and 39.7 in monotherapy and to 36.9 and 32.5 in TCS combination therapy, for BARI 2 mg and BARI 4 mg, respectively by week 16 (), continuing in mean moderate SCORAD range.
Figure 2. Reduction in absolute SCORAD scores in patients treated with BARI monotherapy or TCS combination therapy. Least square mean change from baseline to W16 in absolute SCORAD score in patients from (A) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (B) the TCS combination therapy study (BREEZE-AD7). The proportion of patients achieving SCORAD < 25 through W16 from (C) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (D) the TCS combination therapy study (BREEZE-AD7). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. PBO. BARI: baricitinib; PBO: placebo; SCORAD: SCORing of Atopic Dermatitis; TCS: topical corticosteroid.
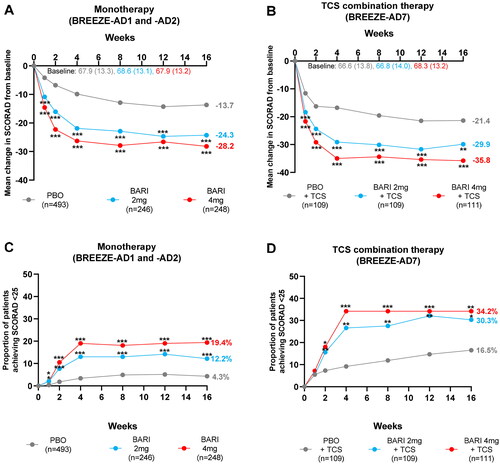
A higher proportion of patients treated with BARI 2 and 4 mg monotherapy achieved SCORAD < 25 compared to PBO as early as W1. For patients treated with BARI 2 and 4 mg TCS combination therapy, significantly more patients achieved SCORAD < 25 compared to patients treated with PBO from W2 onwards ().
Minimal clinically important differences in EASI and SCORAD
MCID for EASI and SCORAD have been calculated as ≥6.6- and ≥8.7-point improvement from baseline, respectively, and represent real changes in disease severity (Citation11). When considering these absolute changes from baseline, significantly more patients treated with BARI 2 and 4 mg monotherapy rapidly achieved an MCID in EASI vs. PBO at W16 (). Similarly, significantly more patients treated with BARI 4 mg TCS combination therapy achieved an MCID in EASI vs. PBO at W16 (). A higher proportion of patients treated with BARI 2 and 4 mg for monotherapy and TCS combination therapy achieved an MCID in SCORAD compared with patients treated with PBO (). Even though SCORAD measures both AD signs and symptoms while EASI measures objective signs only, the proportions of patients achieving an MCID were similar for both EASI and SCORAD.
Figure 3. Stratification of the proportion of patients achieving MCID by severity category. The proportion of patients achieving an EASI MCID of ≥6.6-point improvement from baseline at W16 was stratified by EASI severity category from (A) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (B) the TCS combination therapy study (BREEZE-AD7). The proportion of patients achieving a SCORAD MCID of ≥8.7-point improvement from baseline at W16 was stratified by SCORAD severity category from (C) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (D) the TCS combination therapy study (BREEZE-AD7). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. PBO. BARI: baricitinib; EASI: Eczema Area and Severity Index; MCID: minimal clinically important difference; PBO: placebo; SCORAD: SCORing of Atopic Dermatitis; TCS: topical corticosteroid.
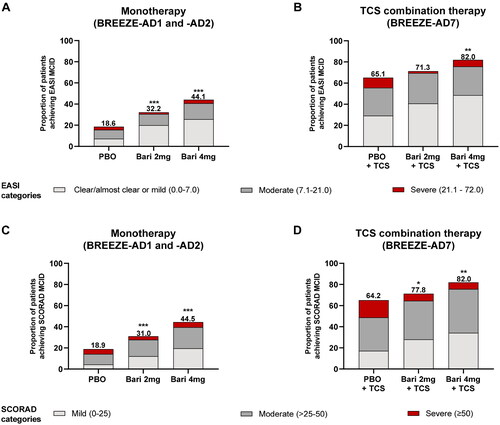
The majority of BARI-treated patients who had reached an MCID in EASI at W16 had achieved EASI ≤ 7. Specifically, 62.0 and 58.3% of patients treated with BARI 2 and 4 mg monotherapy, respectively, compared with 38.9% of patients treated with PBO, and 57.1, 59.3, and 44.9% of patients treated with BARI 2, 4 mg, or PBO in TCS combination therapy presented with EASI ≤ 7 (). Similarly, among patients reaching an MCID in SCORAD at W16, 39.5, 44.0, and 23.1% of patients treated with BARI 2, 4 mg, or PBO monotherapy and 39.3, 41.8, and 26.5% of patients treated with BARI 2, 4 mg, or TCS combination therapy had achieved SCORAD < 25, respectively (). Interestingly, small proportion of patients still presented with the severe disease for both monotherapy and TCS combination therapy based on disease severity categories at W16. This was observed even though they had reached MCID in EASI or SCORAD, potentially reflecting their high disease severity at baseline ().
Improvements in patients’ QoL by objective disease severity
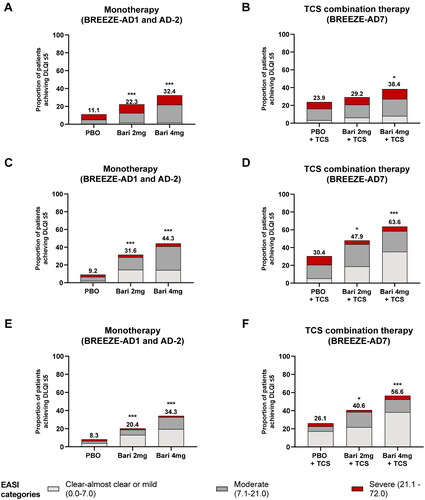
Consistent with previously published data, BARI-treated patients demonstrated rapid improvements in QoL through W16 () (Citation14). When the proportion of patients achieving a DLQI ≤ 5 were stratified by EASI severity category, a small number of BARI-treated patients still had severe disease at W1, W4, and W16, indicating that patients can achieve no/minimal impact of AD on QoL despite residual skin signs. However, the majority of BARI-treated patients achieving a DLQI ≤ 5 at W16 also achieved EASI ≤ 7 ().
Figure 4. The proportion of patients achieving a DLQI ≤ 5 as stratified by EASI severity category. Among patients with a baseline DLQI > 5, the proportion of patients achieving a DLQI ≤ 5 at weeks 1, 4, and 16 was stratified by EASI severity category for (A,C,E) pooled monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and (B,D,F) the TCS combination therapy study (BREEZE-AD7). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. PBO. BARI: baricitinib; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; PBO: placebo; TCS: topical corticosteroid.
Discussion
Relative outcomes are commonly used to assess treatment response over time in RCTs; however, relative outcomes might have limitations in clinical practice and do not inform on actual disease severity at the time of assessment. Therefore, we designed this post-hoc analysis to inform on improvements in AD scores over time in patients treated with BARI monotherapy or TCS combination therapy and to provide an interpretation of these improvements with respect to clinical meaningfulness and patients’ QoL.
Overall, our results show that BARI treatment rapidly improved AD signs and symptoms, when assessed in a composite score. In BARI-treated patients, absolute SCORAD scores decreased rapidly, as did absolute EASI scores when assessing skin signs only. Thereafter, the reduction rate plateaued at W4 and improvements in absolute EASI and SCORAD scores were maintained through W16. The rapid reduction in AD severity was confirmed in clinical practice (Citation19,Citation20) and might be an early indicator as to whether patients respond to BARI treatment.
Patients enrolled in the three BREEZE studies had moderate-to-severe AD as per the studies’ design. However, mean baseline EASI and SCORAD scores indicate predominantly severe disease according to disease severity categories. This might relate to washout periods of 2 and 4 weeks before randomization for topical and systemic therapies in BREEZE trials, respectively, which may have resulted in more severe, active, or flaring disease and might have increased rescue therapy use (Citation21). With BARI treatment, mean EASI and SCORAD rapidly decreased from severe to moderate disease severity categories. Despite high baseline severity, significant proportions of BARI-treated patients achieved EASI ≤ 7 or SCORAD < 25, which encompasses patients achieving clear to almost clear and mild disease. With BARI 4 mg, one in four patients achieved an EASI ≤ 7 response in monotherapy, and one in two in TCS combination therapy after 16 weeks of treatment. Absolute EASI ≤ 7 and SCORAD < 25 were recently proposed, among others, as 6-month treat-to-target goals for patients initiating systemic treatments (Citation12). Our findings thus indicate that BARI treatment is effective and rapid in achieving AD treatment goals, even in more severely affected AD patients. In clinical practice, disease severity might even be lower than in RCTs as patients do not require long washout periods before initiating BARI treatment, and concomitant topical therapies are permitted (Citation19,Citation20,Citation22,Citation23).
Our analyses demonstrated that while many BARI-treated patients achieving an MCID in EASI or SCORAD at W16 had clear/almost clear or mild AD, a small proportion of patients still had severe disease. This might again reflect high disease severity at baseline and suggests that patients with severe AD can experience clinically meaningful improvements before achieving clear/almost clear or mild AD. Interestingly, EASI measures the objective signs of AD only while SCORAD measures both the objective signs and subjective symptoms; however, similar proportions of patients achieved MCIDs in EASI and SCORAD, demonstrating that BARI treatment of AD results in improvements in all aspects contributing to clinical severity in AD. In agreement with this, previous analyses demonstrated that BARI-treated patients reported rapid improvements in itch (measured by itch numeric rating scale, SCORAD-Itch visual analog scale [VAS] and Patient Oriented Eczema Measure [POEM]-itch), itch-associated sleep disturbances (measured by the Atopic Dermatitis Sleep Scale), and overall impact of AD on sleep (measured by SCORAD-Sleep Loss VAS, POEM-Sleep Loss) as early as W1 (Citation24,Citation25). Together, these results indicate that patients in BREEZE-AD1 and -AD2 and BREEZE-AD7, which had the predominantly severe disease at baseline, experienced rapid and sustained improvements in disease severity based on MCIDs for EASI and SCORAD.
Importantly, improvements in skin signs and symptoms were accompanied by rapid improvements in skin-related QoL as early as one week after initiating BARI treatment. Improvements in QoL continued to occur in parallel to skin clearance as most BARI-treated patients achieving a DLQI ≤ 5 at W16 also achieved clear/almost clear or mild skin as measured by EASI. QoL improvements in BARI-treated patients have also been confirmed in clinical practice (Citation19). A small proportion of patients reporting little or no impact of AD on QoL at W16 still had severe skin involvement, which highlights the complex interplay of objective signs and subjective symptoms in patients with AD and the importance of assessing both to fully understand patients’ experiences. Previously published data demonstrated that improvements in disease signs may contribute to some, but not all, improvements in QoL in patients with AD and that itch has a more direct effect on and a higher correlation with DLQI than EASI (Citation25,Citation26). In agreement with this, our analysis demonstrates that at W1, most BARI-treated patients had not yet achieved clear/almost clear or mild skin disease, however, patients still experienced significantly greater improvements in QoL vs. PBO (except patients treated with BARI 2 mg TCS combination therapy) (Citation26). Thus, early improvements in the symptoms of AD extending beyond skin lesions, such as itch, sleep, and functional impairment, may be responsible for improving QoL in patients, and highlight important clinical aspects of AD disease that are not captured by objective instruments, such as EASI, and the importance of AD symptoms for patients’ QoL (Citation14,Citation27).
The importance of using composite scores to assess AD is reflected by current recommendations of the ETFAD (Citation28) and in the guidance from an international treat-to-target consensus recommending the use of PGA and one other disease domain score as the six-month treatment target (Citation12). In agreement with this, the International Eczema Council recommended that the decision to start systemic treatment should include assessments of both disease severity and impact on QoL (Citation29). Our analysis supports the importance of including QoL assessments in disease evaluation and in guiding treatment decisions to enable the full impact of AD on patients’ lives to be considered.
The limitations of this study are that it is a post-hoc analysis, the stratification of EASI, SCORAD, and DLQI by disease severity categories is descriptive only, and there are low numbers of patients for some of these categories.
Overall, our results demonstrate that BARI monotherapy and TCS combination therapy provided rapid and sustained improvements in both the signs and symptoms of AD, with a significant proportion of patients achieving clear/almost clear or mild disease based on EASI and SCORAD severity categories despite high disease burden at baseline. This was accompanied by rapid QoL improvements, which were not adequately reflected in skin severity, indicating that objective evaluations using physician-assessed scores do not necessarily correlate with the patient’s impression of AD improvement.
Acknowledgments
The authors would like to thank Gabrielle Stack, PhD and Joyce O’Grady, PhD, medical writers and employees of Eli Lilly and Company, for writing and editorial support.
Disclosure statement
Jacob P. Thyssen has been a consultant for and/or has received grant/research/honorarium support from: Regeneron, Sanofi-Genzyme, LEO Pharma, AbbVie, Eli Lilly and Company, Pfizer. Thomas Bieber was speaker and/or consultant and/or Investigator for AbbVie, Affibody, Almirall, AnaptysBio, Arena, Asana Biosciences, ASLAN pharma, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Connect Pharma, Dermavant, Domain Therapeutics, EQRx, Galderma, Glenmark, GSK, Incyte, Innovaderm, IQVIA, Janssen, Kirin, Kymab, LEO, LG Chem, Lilly, L’Oréal, MSD, Novartis, Numab, OM-Pharma, Pfizer, Pierre Fabre, Q32bio, RAPT, Sanofi/Regeneron, UCB. He is founder and chairman of the board of the non-profit biotech “Davos Biosciences”. C. Elise Kleyn’s conflicts of interest are as follows (including consulting fees, research or institutional support, and educational grants): Almirall, Amgen, Celgene, Eli Lilly and Company, Janssen Pharmaceuticals, La Roche-Posay, LEO Pharma, Novartis, Pfizer, and UCB. Audrey Nosbaum has received grants as an investigator and honoraria for lecturing, or consulting fees from: AbbVie, Celgene, Eli Lilly and Company, Galderma, Janssen Cilag, LEO Pharma, Medac, Novartis, Pierre Fabre, Pfizer, and Sanofi-Regeneron. Susanne Grond is an employee and minor shareholder of Eli Lilly and Company. The study was sponsored by Eli Lilly and Company, under license from Incyte Corporation. Helmut Petto was an employee of Eli Lilly and Company during the development of the publication. Elisabeth Riedl was an employee of Eli Lilly and Company during the development of the publication and has received honoraria for lecturing from Eli Lilly and Company. Her affiliation is Medical University Vienna. Andreas Wollenberg has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Beiersdorf, Bioderma, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Galapagos, Galderma, Janssen-Cilag, LEO, Loreal, Eli Lilly, Novartis, Pfizer, Pierre Fabre, Regeneron, and Sanofi.
Data availability statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://vivli.org/.
Additional information
Funding
References
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1–9.
- Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120(1):10–22.e2.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134(6):1527–1534.
- Iannone M, Tonini G, Janowska A, et al. Definition of treatment goals in terms of clinician-reported disease severity and patient-reported outcomes in moderate-to-severe adult atopic dermatitis: a systematic review. Curr Med Res Opin. 2021;37(8):1295–1301.
- Chopra R, Vakharia PP, Sacotte R, et al. Relationship between EASI and SCORAD severity assessments for atopic dermatitis. J Allergy Clin Immunol. 2017;140(6):1708–1710.e1.
- Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
- Leshem YA, Hajar T, Hanifin JM, et al. What the eczema area and severity index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353–1357.
- Schram ME, Spuls PI, Leeflang MM, et al. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106.
- De Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402.
- Thyssen JP, Vestergaard C, Deleuran M, et al. European Task Force on Atopic Dermatitis (ETFAD): treatment targets and treatable traits in atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(12):e839–e842.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6):691–699.
- European Medicines Agency Olumiant: EPAR – Product Information; 2021.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664.
- Rogner D, Biedermann T, Lauffer F. Treatment of atopic dermatitis with baricitinib: first real-life experience. Acta Derm Venereol. 2022;102:adv00677.
- Uchiyama A, Fujiwara C, Inoue Y, et al. Real-world effectiveness and safety of baricitinib in Japanese patients with atopic dermatitis: a single-center retrospective study. J Dermatol. 2022;49(4):469–471.
- Silverberg JI, Simpson, EL, Armstrong AW, et al. Expert perspectives on key parameters that impact interpretation of randomized clinical trials in moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2022;23(1):1–11.
- Heratizadeh A, Haufe E, Stölzl D, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol. 2020;34(6):1263–1272.
- Ariëns LFM, van der Schaft J, Bakker DS, et al. Dupilumab is very effective in a large cohort of difficult-to-treat adult atopic dermatitis patients: first clinical and biomarker results from the BioDay registry. Allergy. 2020;75(1):116–126.
- Buhl T, Rosmarin D, Serra-Baldrich E, et al. Itch and sleep improvements with baricitinib in patients with atopic dermatitis: a post-hoc analysis of 3 phase 3 studies. Dermatol Ther. 2021;11(3):971–982.
- Reich K, DeLozier AM, Nunes FP,et al., Baricitinib improves symptoms in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: patient-reported outcomes from two randomized monotherapy phase III trials. J Dermatolog Treat. 2022;33(3):1521–1530.
- Yosipovitch G, Papp K, Forman S, et al. The contribution of itch and skin severity improvements to the dermatology life quality index in patients with atopic dermatitis in baricitinib phase III trials. Br J Dermatol. 2022;186(6):1047–1049.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35(7):1543–1552.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744.
- Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77(4):623–633.

