Dear Editor,
Atopic dermatitis (AD) is the most prevalent inflammatory cutaneous disease worldwide, affecting up to 20% of children and 3% of adults (Citation1). According to the most recent European Guidelines, cyclosporine, dupilumab, tralokinumab, upadacitinib, baricitinib, and abrocitinib are all recommended for moderate-to-severe cases of AD in adult patients (Citation2), with dupilumab and upadacitinib being also approved in adolescents (Citation3). Tralokinumab is the first selective inhibitor of interleukin (IL)-13 approved for treating AD after being evaluated in several Phase-III clinical trials (Citation4,Citation5). However, limited data are currently available regarding the effectiveness and safety profiles of tralokinumab in a real-world setting.
We retrospectively analyzed data of 10 patients followed at our Dermatology Unit, all treated with tralokinumab for at least 16 weeks (). All patients received tralokinumab according to the summary of product characteristics (Citation6). At baseline and week 16, demographic characteristics, along with EASI (Eczema Area and Severity Index), pp-NRS (peak pruritus Numerical Rating Scale), s-NRS (sleep Numerical Rating Scale), and ADCT (Atopic Dermatitis Control Tool) scores, were all recorded. To evaluate the impact of tralokinumab on the patient’s quality of life, at week 16, the following endpoints were assessed: the achievement of ADCT ≤7, a decrease of at least 4 points of pp-NRS and s-NRS compared with baseline (). Moreover, the clinical effectiveness of tralokinumab was evaluated in terms of EASI 50 and EASI 75 (reduction of at least 50% and 75% in EASI compared with baseline, respectively) and Investigator’s Global Assessment (IGA) 0/1 (clear or almost clear) (). During the treatment, patients were allowed to apply medium-potency TCS if needed. Data are expressed as absolute frequencies and percentages, or as median and interquartile range (IQR), as appropriate.
Figure 1. Impact of the treatment with tralokinumab on subjective symptoms and patients’ quality of life: achievement of pp-NRS and s-NRS reduction ≥ 4 points (a) and ADCT ≤ 7 (c) at week 16. Decrease in median pp-NRS and s-NRS (b) and median ADCT (d) after 16 weeks. pp-NRS: Peak Pruritus- Numerical Rating Scale; s-NRS: Sleep-Numerical Rating Scale; ADCT: Atopic Dermatitis Control Tool.
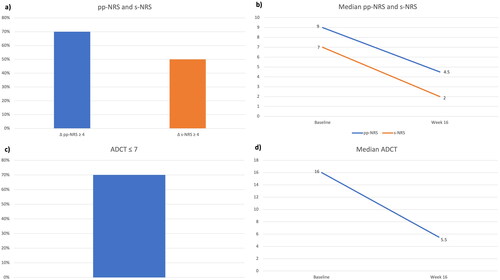
Figure 2. Clinical improvement after 16 weeks of treatment with tralokinumab: decrease in median EASI (a); percentages of patients achieving EASI 50 and EASI 75 (b), and proportion of patients reaching an IGA of 0 or 1 (c) at week 16. EASI: Eczema Area and Severity Index; IGA: Investigator’s Global Assessment.
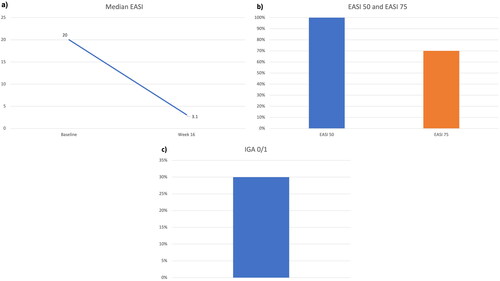
Table 1. Demographic and clinical characteristics of our patients at baseline.
At baseline, seven patients (70%) were females, and the median age was 32.5 years (IQR= 23.75). Four patients (40%) were affected by adult-onset AD (≥ 20 years), and the median BMI was 21.70 (IQR= 6.44). The most frequent comorbidities were allergic rhinitis (three patients) and asthma (two). All patients had previously failed cyclosporine, and half of them (50%) had received dupilumab, with only partial effectiveness. At baseline, patients reported a median ADCT of 16 (IQR= 2.75), median pp-NRS of 9 (1.5) and a median s-NRS of 7 (1.1). Regarding clinical scores, the median EASI at baseline was 20 (IQR= 8), with all patients having at least an IGA of 3. After 16 weeks, eight patients (80%) achieved an ADCT ≤7, with a median ADCT of 5.5 (IQR= 2). The pp-NRS also improved dramatically, as a decrease of ≥4 points was observed in seven patients (70%), with a median pp-NRS of 4.5 (IQR= 3). Five patients (50%) showed a ≥ four-point reduction in s-NRS, and the median s-NRS at week 16 was 2 (IQR= 4). Regarding clinical outcomes, the mean EASI decreased to 3.1 (IQR= 3.35), with 10 (100%) and 7 patients (70%) achieving EASI 50 and EASI 75, respectively. Three patients (30%) reached an IGA of 0/1 at week 16. No significant safety findings were observed, as only one patient experienced nasopharyngitis, and no discontinuations due to adverse events were recorded.
Our experience showed that the treatment with tralokinumab significantly impacts patients’ quality of life and symptoms after just 16 weeks. Compared with Phase-III clinical trials, we observed better effectiveness on itch, as a pp-NRS reduction ≥ 4 was observed in 70% of our compared versus 20% and 25% in the ECZTRA1 and two trials (Citation4). The same endpoint was reached by 35.4% of patients in the ECZTRA3 trial (Citation5) (which allowed the concomitant use of TCS) at week 16. Regarding EASI 75, we observed better results than the ECZTRA3 trial and a recent real-world experience (Citation7) (70% versus 57.6% and 56%). Our patients achieved comparable or slightly better rates of IGA 0/1 at week 16 compared with all phase-III clinical trials. The safety profile in our experience was consistent with data from the aforementioned clinical trials (Citation4,Citation5). No cases of conjunctivitis, laboratory abnormalities, facial redness, and herpes infections were observed, differently from available real-world data for other systemic drugs for AD (Citation8,Citation9). Despite a limited sample size, our study supports the use of tralokinumab in real-world patients with AD, highlighting a strong impact on AD symptoms and patients’ quality of life and showing effectiveness regarding all clinical endpoints after just 16 weeks.
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy
Luciano Ibba
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy
[email protected]
Carlo Alberto Vignoli
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy
Paola Facheris
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy
Mario Valenti and Antonio Costanzo
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, Italy; Department of Biomedical Sciences, Humanitas University, Milan, Italy
Alessandra Narcisi
Dermatology Unit, IRCCS Humanitas Research Hospital, Milan, ItalyReceived 9 May 2023; accepted 11 May 2023© 2023 The Author(s). Published with license by Taylor & Francis Group, LLC
Ethical approval
Institutional review board approval was exempted, as the study procedures did not deviate from standard clinical practice. All included patients had provided written informed consent for the retrospective analysis of their clinical data. The study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Disclosure statement
L. Gargiulo has been a consultant for Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie, and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen, and Boehringer Ingelheim. L. Ibba, P. Facheris, and C. A. Vignoli have nothing to declare.
Data availability statement
Additional data supporting the findings of this manuscript are available on reasonable request to the corresponding author.
Additional information
Funding
References
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66 Suppl 1:1–3.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431.
- Gargiulo L, Vignoli CA, Ibba L, et al. Real-life effectiveness and safety of dupilumab in adolescents with atopic dermatitis: a 52-week single-center retrospective study. J Dermatolog Treat. 2023;34(1):2200867.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449.
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–463.
- European Medicines Agency. Adtralza (tralokinumab): summary of product characteristics. 2021 [cited 2023 May 02]. Available from: https://www.ema.europa.eu/en/documents/product-information/adtralza-epar-product-information_en.pdf
- Pereyra-Rodriguez JJ, Herranz P, Ruiz-Villaverde R, et al. Treatment of severe atopic dermatitis with tralokinumab in real clinical practice. Short-term effectiveness and safety results. Clin Exp Dermatol. 2023 [cited 2023 Apr 25]; llad153.
- Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther. 2023;13(2):651–660.
- Touhouche AT, Cassagne M, Bérard E, et al. Incidence and risk factors for dupilumab associated ocular adverse events: a real-life prospective study. J Eur Acad Dermatol Venereol. 2021;35(1):172–179.

