Abstract
Background
Pharmacoeconomic studies examining the cost-effectiveness of adalimumab biosimilars versus methotrexate in real-life settings are limited.
Objectives
To assess the cost per responder from the perspective of the National Health System of adalimumab biosimilars versus methotrexate for psoriasis treatment in a real-life setting.
Methods
A cost-per responder analysis comparing adalimumab biosimilars MSB11022 (Idacio®) and ABP 501 (Amgevita) versus subcutaneous methotrexate was performed. The incremental cost per responder was calculated by multiplying the cost of treatment (including the discounts, as published in the framework agreement of the Veneto region) and the number needed to treat each therapy. The clinical efficacy measures were defined as being on treatment (i.e., retention rate) at weeks 24 and 52.
Results
A total of 712 adult patients with moderate-to-severe chronic plaque psoriasis consecutively admitted to the outpatient clinic from January 2021 to December 2022 were included; 160 were treated with ABP 501 (Amgevita), 250 with MSB11022 (Idacio) and 302 with methotrexate. The retention rates of Amgevita, Idacio and methotrexate at week 24 were 86%, 90% and 78%, and 81%, 82% and 63% at week 52, respectively. The cost per responder at week 24 was €674 for Amgevita, €366 for Idacio and €264 for methotrexate, respectively; at week 52, was €1430 for Amgevita® €799 for Idacio® and €652 for methotrexate, respectively.
Conclusions
The real-life cost-effectiveness of biosimilar drugs is largely influenced by discount rates. The week 52 cost-effectiveness of Idacio is comparable to subcutaneous methotrexate. The lowering of the cost of biosimilar drugs makes them a more accessible therapeutic option and they also can be introduced earlier in the treatment of moderate-to-severe psoriasis.
Introduction
Psoriasis is a common chronic immune-mediated disease that affects 1–4% of the Western population, and about 14 million people in Europe [Citation1]. About 20–30% of psoriatic patients have a moderate-to-severe form of the disease [Citation2,Citation3] and are candidate to phototherapy or to systemic treatments such as conventional systemic agents (acitretin, cyclosporine, methotrexate, fumarates) and targeted therapies (biologics and small molecules) [Citation4]. The treatment of moderate-to-severe psoriasis with biologic agents poses a significant economic burden to the health systems [Citation1,Citation2,Citation5,Citation6]. Psoriasis can be associated with several comorbidities, including PsA, cardiovascular diseases, metabolic syndrome, inflammatory bowel diseases and psychiatric diseases [Citation7]. Among biological treatments, adalimumab belongs to the class of TNF-α inhibitors. In the last few years, the expiration of biologic patents of some TNF-α inhibitors, has encouraged the production of biosimilars, with a very similar safety and efficacy profile, but a much lower cost compared to their originator. The use of biosimilars is a valuable pharmacoeconomic strategy to lower healthcare cost and can effectively lead to early introduction of safer and more effective treatment of psoriasis [Citation8]. The early intervention with biosimilars may also prevent progression to PsA, with a more persistent efficacy and may modify the natural history of psoriasis [Citation9–11]. The objective of this study was to compare the cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis from the perspective of the Italian National Health System.
Material and methods
The study population included adult patients with moderate-to-severe chronic plaque psoriasis consecutively admitted to the outpatient clinic of the University Hospital of Verona from January 2021 to December 2022 (). A cost per responder analysis of MSB11022 (Idacio), ABP 501 (Amgevita) biosimilars versus subcutaneous methotrexate was developed based on real life experience. A responder was defined as a patient with an improvement in psoriasis area severity index (PASI) consistent with the definition of success with the primary efficacy variable. In detail, a responder is a patient where the achievement of PASI 75 was meant. The clinical efficacy measures were defined as being on treatment (i.e., retention rate) at week 24 and 52. All the patients of this study are naïve to systemic treatments for moderate-to-severe psoriasis, thus none of the patients failed systemic treatments previously and none of the patients have been switched to other systemic treatments previously. In addition, all patients were treated as monotherapy.
Table 1. Characteristics of study population (patients treated with adalimumab and methotrexate).
Drug administration scheme
Dosing regimens for adalimumab biosimilars 40 mg were those labeled for moderate-to-severe chronic plaque psoriasis in adults [Citation12], i.e., subcutaneous injections 80 mg at week 0, then 40 mg every other week starting at week 1. Methotrexate was administered at dose ranging from 10 to 15 mg weekly with subcutaneous injection with a mean ± standard deviation weekly dosages of 12.8 ± 2.0 mg. The costs of folic acid, at the guideline recommended weekly oral dose of 5 mg, were also added to the costs of methotrexate treatment.
Cost per responder model
The cost per responder model was based on the perspective of the Italian National Healthcare System. Regarding cost of biologic drugs, ex-factory wholesale purchase prices were used, including the mandatory discounts according to the national legislation (5% discount, plus a further 5% reduction on the discount result) and the additional discounts applied from the pharmacy of the hospital that is published in the current NNT framework agreement of the Veneto region [Citation13–15] (). For methotrexate and folic acid, official retail prices were used. All costs were reported as for 2023 in Euro. Only drug acquisition costs were considered, while other costs including treatment administration, visits and laboratory monitoring were excluded. The incremental cost per responder was calculated by multiplying the cost of treatment and number needed to treat (NNT) for each of the therapies. In detail, the NNT has been derived from the arithmetic division between 100 and the percentage of the retention rate at week 24 and at week 52. This number derived, the NNT, was multiplied by the corresponding weeks of treatment (24 and 52 weeks), to obtain the incremental cost per responder for each drug ().
Table 2. Drug costs of adalimumab biosimilars, methotrexate and folic acid according to list price (in Euro).
Table 3. Retention rate at week 24 and 52 in the study population (patients treated with adalimumab and methotrexate).
Retention rate
The retention rate of a biological drug is the percentage of patients remaining on treatment over time and provides an index of a drug’s overall effectiveness. Drug survival tells us that if the drug is continued it means that it is satisfactorily effective, tolerated, and safe. The overall retention rate of Amgevita, Idacio and methotrexate were calculated based on the Kaplan–Meier curve, which reflects the median disease duration. The disease duration refers to the beginning of therapy with the event being the treatment discontinuation.
Results
The study population consisted of 712 patients; in particular, 160 were treated Amgevita, 250 with Idacio, and 302 with methotrexate. The characteristics of the patients, including presence or the absence of comorbidities are summarized in . The three group of patients did not differ significantly as for all the variables listed, including disease severity and the presence of psoriatic arthritis (p < 0.001). The percentage of censored patients is very low (0.2%, due to the patient’s death), as we are a university hospital and one of the few centers involved in the dispensation of biological drugs for the treatment of psoriasis.
The incremental cost per responder was calculated by multiplying the cost of treatment and number needed to treat (NNT) for each of the therapies. In particular, the NNT has been derived from the arithmetic division between 100 and the percentage of the retention rate at week 24 and at week 52. This number derived, the NNT, was multiplied by the corresponding weeks of treatment (24 and 52 weeks), to obtain the incremental cost per responder for each drug. The retention rate of Amgevita, Idacio and methotrexate at week 24 were 86% (CI 95% 79–91), 90% (CI 95% 86-94) and 78% (CI 95% 72–85), respectively. At week 52, the retention rate of Amgevita, Idacio and methotrexate were 81% (CI 95% 74–86), 82% (CI 95% 76–84) and 63% (CI 95% 58–76), respectively. The cost per responder analysis based on the retention rate at week 24 and 52 in patients receiving adalimumab biosimilars (Amgevita and Idacio) or methotrexate 12.5 mg plus folic acid 5 mg, is reported in and . The cost per responder calculated based on the ex-factory price was €6941 for Amgevita, €5894 for Idacio, and €264.59 for methotrexate 12.5 mg, at week 24; €14,720.30 for Amgevita, €12852 for Idacio, and €652 for methotrexate 12.5 mg, at week 52. Considering the final discounted price, which reflect the real biosimilars cost, the cost per responder was €674 for Amgevita, €366 for Idacio, and €264 for methotrexate at week 24. At week 52, the cost per responder was €1430 for Amgevita €799 for Idacio and €652 for methotrexate, respectively (). Different scenario analyses were undertaken assuming alternative drug acquisition discounts (–40%; −60%; −80%) (Supplementary Tables).
Figure 1. Cost per responder analysis for patients receiving ABP 501 (Amgevita®) (black histogram), MSB11022 (Idacio®) (grey histogram) and methotrexate (white histogram) at week 24.
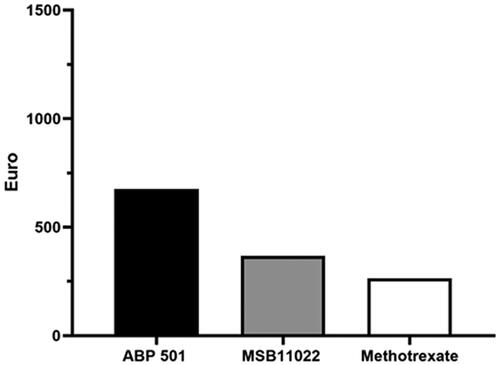
Figure 2. Cost per responder analysis for patients receiving ABP 501 (Amgevita®) (black histogram), MSB11022 (Idacio®) (grey histogram) and methotrexate (white histograms) at week 52.
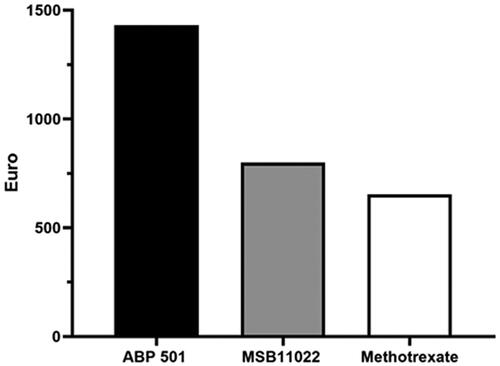
Table 4. Cost per responder of adalimumab biosimilars and methotrexate at week 24 and 52 (in Euro).
Discussion
The present study is a real-life experience that compared the economic value, using a cost per responder analysis, of adalimumab biosimilars 40 mg (Amgevita and Idacio) and methotrexate 12.5 mg plus folic acid 5 mg. The cost per responder analysis indicated that only one biosimilar (Idacio®) approached the costs per responder of methotrexate. Our analysis did not include the cost of visits and laboratory test, but overall, they are similar between adalimumab and methotrexate. The better cost-effectiveness of biosimilars may allow a higher number of patients with moderate-to-severe psoriasis to receive a biological treatment. Indeed, a significant proportion of patients with moderate-to-severe psoriasis is not receiving an adequate treatment also for the high cost of drugs, and many patients with moderate-to-severe psoriasis are still treated only with topical agents [Citation16–18]. The considerable reduction in the cost gap between adalimumab biosimilars and methotrexate may prompt their early use. Indeed, methotrexate is associated with a number of tolerability and safety issues, which represent common cause of drug discontinuation, and affect the long-term retention rate of the drug [Citation19–21]. Among these, methotrexate is associated with a significant risk of hepatotoxicity, possibly because fatty liver disease is quite common in patients with plaque psoriasis [Citation22–24]. In a population-based cohort study, mild liver disease and cirrhosis-related hospitalization had an incidence rate per 1000 person-years of 4.22 (95% confidence interval-CI 3.61–4.91) and 0.73 (95% CI 0.49–1.05) in patients treated with methotrexate, respectively [Citation25]. Also important to consider is that methotrexate is teratogenic in women and also contraindicated in men who want to become fathers.
There are limitations to this study. The study is in the perspective of the payer, and it must be considered that the dispensing of the biosimilars is free for the patient, while for methotrexate is partly borne by the patient. Given the chronic nature of psoriatic disease and its long-term therapy, it is important to investigate the economic value of the different systemic agents as even small differences in costs can be meaningful over a long-time horizon. In addition, the study is in the perspective of the Italian market. To overcome this issue, we included a scenario analysis with discount percentages of 40%, 60% and 80% which, however, provide consistent results. The present study highlighted that, from the perspective of the Italian National Health Service, the economic impact of adalimumab biosimilars is comparable to the methotrexate, especially for Idacio, and in particular at the week 52; the safety and tolerability profile for a long-term treatment is in favor of the biosimilars. Another limitation is that, since it is a real-life study, the groups may be imbalanced with respect to severity and other forms of selection bias.
In conclusion, adalimumab biosimilar offer a cheap treatment option for patients with moderate-to-severe psoriasis. The possibility of treating patients earlier with an effective and safe treatments is relevant to possibly modify the disease course and prevent the develop of psoriatic arthritis [Citation11,Citation26–28].
Supplemental Material
Download PDF (77.1 KB)Disclosure statement
M. Maurelli has no conflict of interest to disclose; P. Gisondi has been consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Leo-pharma, Eli Lilly, Novartis, Pierre Fabre, Sandoz, Sanofi and UCB; G. Girolomoni served as consultant and/or speaker for AbbVie, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli-Lilly, Leo Pharma, Merck Serona, Novartis, Pfizer, Regeneron, Samsung bioepis, Sanofi and UCB.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Gisondi P, Geat D, Armeni P, et al. Cost per responder of adalimumab biosimilars MSB11022 and ABP 501 versus the originator and methotrexate in chronic plaque psoriasis. Expert Opin Biol Ther. 2022;26:1–5.
- Gisondi P, Geat D, Maurelli M, et al. Cost per responder analysis of secukinumab versus adalimumab in the treatment of psoriatic disease. Vaccines. 2022;10(5):646.
- Colombo G, Altomare G, Peris K, et al. Moderate and severe plaque psoriasis: cost-of-illness study in Italy. Ther Clin Risk Manag. 2008;4(2):559–568.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris-update 2015-short version-EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294.
- Bellinato F, Gisondi P, Mason E, et al. Real-Life effectiveness of adalimumab biosimilars in patients with chronic plaque psoriasis. Dermatol Ther. 2022;12(6):1303–1311.
- Esposti LD, Perrone V, Sangiorgi D, et al. Analysis of drug utilization and health care resource consumption in patients with psoriasis and psoriatic arthritis before and after treatment with biological therapies. Biologics. 2018;12:151–158.
- Gisondi P, Bellinato F, Maurelli M, et al. Reducing the risk of developing psoriatic arthritis in patients with psoriasis. Psoriasis. 2022;12:213–220.
- Vogler S, Schneider P, Zuba M, et al. Policies to encourage the use of biosimilars in European countries and their potential impact on pharmaceutical expenditure. Front Pharmacol. 2021;12:625296.
- Gisondi P, Geat D, Conti A, et al. TNF-α inhibitors biosimilars as first line systemic treatment for moderate-to-severe chronic plaque psoriasis. Expert Rev Clin Immunol. 2020;16(6):591–598.
- Gisondi P, Altomare G, Ayala F, et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2017;31(5):774–790.
- Gisondi P, Bellinato F, Targher G, et al. Biological disease-modifying antirheumatic drugs may mitigate the risk of psoriatic arthritis in patients with chronic plaque psoriasis. Ann Rheum Dis. 2022;81(1):68–73.
- Nast A, Smith C, Spuls PI, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris - Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498.
- Available from: https://www.gazzettaufficiale.it/eli/id/2016/11/11/16A07913/sg.
- Available from: https://www.gazzettaufficiale.it/eli/id/2021/07/28/21A04520/SG#:∼:text=Prezzo%20al%20pubblico%20(iva%20inclusa,E%20(in%20base%2010.)
- Available from: http://azero.veneto.it/public/alboOnline.xhtml?pageType=all.
- Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the national psoriasis foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185.
- Burgos-Pol R, Martínez-Sesmero JM, Ventura-Cerdá JM, et al. The cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic review. Actas Dermosifiliogr. 2016;107(7):577–590.
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70(5):871–881.
- Sbidian E, Chaimani A, Afach S, et al. L. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1(1):CD011535.
- Ozkok Akbulut T, Topaloglu Demir F, Oguz Topal I, et al. Drug survival and predictor factors for discontinuation of methotrexate in psoriasis: a real-life multicenter study. Int J Dermatol. 2021;60(9):1140–1147.
- Mourad AI, Gniadecki R. Biologic drug survival in psoriasis: a systematic review & comparative meta-analysis. Front Med. 2020;7:625755.
- Whiting-O’Keefe QE, Fye KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90(6):711–716.
- Mantovani A, Gisondi P, Lonardo A, et al. Relationship between non-alcoholic fatty liver disease and psoriasis: a novel hepato-dermal axis? Int J Mol Sci. 2016;17(2):217.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656–662.
- Gelfand JM, Wan J, Zhang H, et al. Risk of liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis receiving methotrexate: a population-based study. J Am Acad Dermatol. 2021;84(6):1636–1643.
- Acosta Felquer ML, LoGiudice L, Galimberti ML, et al. Treating the skin with biologics in patients with psoriasis decreases the incidence of psoriatic arthritis. Ann Rheum Dis. 2022;81(1):74–79.
- von Stülpnagel CC, Augustin M, Düpmann L, et al. Mapping risk factors for cumulative life course impairment in patients with chronic skin diseases – a systematic review. J Eur Acad Dermatol Venereol. 2021;35(11):2166–2184.
- Iversen L, Eidsmo L, Austad J, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification - rationale and design of the randomized, multicenter STEPIn study. J Eur Acad Dermatol Venereol. 2018;32(11):1930–1939.

