Abstract
Background
Dupilumab facial redness (DFR) is a side effect of dupilumab treatment that has only been recently reported. We previously reported on two patients with DFR who were successfully treated with a topical formulation containing human adipose tissue-derived mesenchymal stem cell-derived exosomes (ASCEs).
Objectives
The study aimed to evaluate the efficacy and safety of ASCEs in DFR.
Participants and methods
We performed 12-week prospective study at single center. Twenty adult atopic dermatitis patients diagnosed with DFR were enrolled. They were treated with a topical application of the exosome formulation every week for five consecutive weeks.
Results
After exosome treatment, both the average investigator global assessment score and clinical erythema assessment scale scores decreased. 19 patients (95%) were satisfied with the treatment. Compared to baseline, erythema index at week 4 were decreased by 31, 27, 13, and 25 units on the forehead, chin, right and left cheek respectively. The analysis of stratum corneum samples revealed the expression of IL-1α and human thymic stromal lymphopoietin was suppressed after exosome treatment, whereas filaggrin and vascular endothelial growth factor expression increased.
Conclusions
This study suggests topical formulation containing ASCEs can alleviate DFR by downregulating local inflammation and restoring skin barrier function.
Introduction
Dupilumab facial redness (DFR) is a recently reported side effect of dupilumab treatment for atopic dermatitis (AD). Although it was not reported as an adverse event in phase 3 clinical trials, recent studies reported DFR occurred in approximately 4–10% of patients treated with dupilumab (Citation1–7). Erythema on visible areas including face and neck leads to a profound psychosocial burden and some patients discontinue dupilumab treatment because of this adverse event (Citation1). Unfortunately, the etiology and treatment of this condition remain elusive (Citation8,Citation9).
Exosome treatment is a recently developed cell-free therapeutic strategy in regenerative medicine. Because of their small size (nanosize), biological origin, lipid bilayer membrane, ability to communicate intracellularly, and capacity to modulate the molecular activities of the recipient cell, they are attracting significant interest among dermatologists (Citation10,Citation11). Exosomes from mesenchymal stem cells (MSCs) are highly immunomodulatory and have shown therapeutic effects regarding wound healing, prevention of scar, skin pigmentation, and wrinkles as well as improving chronic inflammatory skin disorders such as psoriasis, AD, systemic lupus erythematosus, or bullous pemphigoid (Citation11–23). Previously, we reported two successful cases of DFR treated with topical application of human adipose tissue-derived mesenchymal stem cell (MSC)-derived exosomes (ASCEs) (Citation24). In this study, we aimed to verify the efficacy of an ASCE topical formulation in treating DFR.
Materials and methods
Study design and subjects
This was a 12-week prospective study, approved by the Chung-Ang University Hospital Institutional Review Board (IRB no. 2109-014-477). Twenty Korean patients aged 18 and older, who were on dupilumab for AD and were diagnosed with DFR in Chung-Ang University Hospital (Seoul), were recruited in the study. The patients in this manuscript participated in the study voluntarily with good compliance and had given written informed consent to publication of their clinical data and photographs. The treatment and examination took 30 min to an hour.
Exosome treatment
We used the exosome formulation ASCE + SRLV-S (ExoCoBio Inc., Seoul, Republic of Korea), which contains 20 mg (970,000 ppm) of lyophilized human ASCE. Exosomes in this product were acquired from a human adipose-derived stem cell (ASC)-conditioned medium (CM) developed by ExoSCRTTM technology as described previously (ExoCoBio Inc., Seoul, Republic of Korea) (Citation24,Citation25). Briefly, ASC-CM was collected from ASCs cultured in serum-free Dulbecco’s Modified Eagle’s Medium (Thermo Fischer Scientific, Waltham, MA, USA), and the non-exosomal particles were removed by a 0.2 μm filter. Next, the exosomes were further purified and concentrated through tangential-flow filtration, and quantification was performed by nanoparticle tracking analysis. Characterization of ASCEs used in this study is described in Supplementary Figure 1.
One vial of ASCE + SRLV-S was applied to each patient’s entire face by dermatologists, and prism sonophoresis (Alummedi, Seoul, Korea) was used to ensure effective drug delivery. This topical application of exosomes was performed weekly for five consecutive weeks.
Clinical evaluation and measurement of skin parameters
Clinical and objective evaluations were performed at baseline and every week before the ASCE treatment and then at weeks 8 and 12 (four and eight weeks after the final exosome treatment). Clinical photographs were taken using the Mark-Vu® instrument (PSI plus Co. Ltd.) at each visit. The data from an investigator global assessment (IGA, 0–5, ), a clinical erythema assessment scale (CEA, 0–4, ), and subjective satisfaction (1–5, ) were recorded. Skin erythema, skin hydration, and trans-epidermal water loss (TEWL) were measured using Mexameter® MX18, Corneometer® CM825, and Tewamater® TM300 (Courage & Khazaka GmbH, Cologne, Germany). Measurements were taken in accordance with the manufacturer’s guidelines at the forehead, chin, right cheek, and left cheek. During the measurements, the room temperature was maintained at a constant 20–22 °C and a relative humidity range of 40–60%.
Table 1. Rating grade for investigator global assessment score.
Table 2. Rating grade for clinical erythema assessment scale.
Table 3. Rating grade for patient satisfaction.
Analysis of stratum corneum samples
At baseline and week 8 (four weeks after final exosome treatment), stratum corneum samples were collected using tape stripping (skin D-squame), and subsequently analyzed in order to evaluate inflammatory cytokines and skin barrier function. Quantitative analysis of interleukin-1α (IL-1α), human thymic stromal lymphopoietin (TSLP), filaggrin (FLG), and vascular endothelial growth factor (VEGF) was performed through real-time-polymerase chain reaction (RT-PCR) and Western blot techniques. The detailed experimental method can be found in Supplementary Material 1.
Statistical analysis
We compared the resulting metabolic activities of the treatment groups and controls using one-way analysis of variance (ANOVA) and Tukey’s multiple-comparison posttest. Differences between groups were considered to significant at a p value of <0.05. Statistical analyses were performed with GraphPad Prism 8.0 (GraphPad Software, Inc., CA, USA).
Results
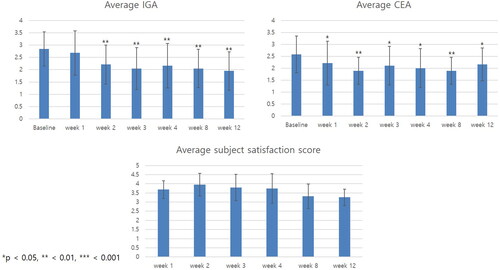
Investigator global assessment, clinical erythema assessment scale, and subjective satisfaction score
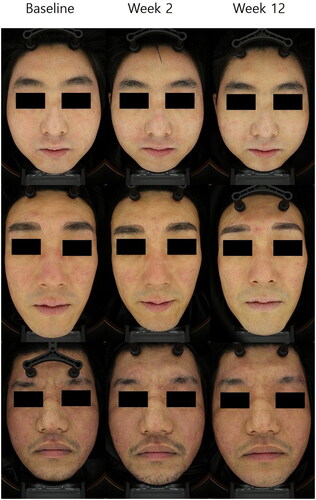
The average IGA score decreased from week 2 and continued to decrease until week 12 (). Overall, 12 out of 20 (60%) patients achieved an IGA score of 1 (almost clear) at least once during the entire clinical trial period. Similarly, the average CEA score decreased from the first week after the first exosome treatment. A total of 11 out of 20 (55%) patients achieved a CEA score of 1 at least once during the entire clinical trial period. The average subject satisfaction score was highest in week 2 and slowly decreased over time. However, the average score was above 3 (fair) at all times. Altogether, 19 out of 20 (95%) patients had a subjective satisfaction score of 4 (satisfied) at least once during the entire clinical trial period. Representative photographs of three patients at baseline, after the first treatment, and at week 12 are shown in .
Figure 1. Clinical outcomes of patients with dupilumab facial redness treated with adipose-derived stem cell exosome. Change in average investigator global assessment (IGA), clinical erythema assessment (CEA), and patient satisfaction score over time. The average IGA score decreased from week 2 and continued to decrease until week 12. The average CEA score decreased significantly from the first week after the first exosome treatment. Overall, 19 out of 20 (95%) patients had a subjective satisfaction score of 4 (satisfied) at least once during the entire clinical trial period. *p < 0.05, **<0.01, ***<0.001, compared with baseline.
Skin erythema, hydration, and trans-epidermal water loss
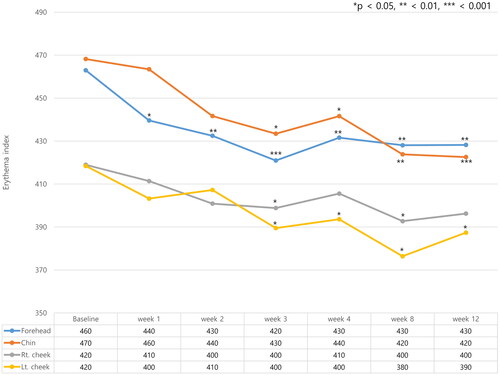
Skin erythema was measured at the forehead, chin, right cheek, and left cheek. Erythema index (EI) decreased in all four areas after exosome treatment and decreased fastest on the forehead, where it decreased from week 2. EI on the forehead was 460 at baseline, which decreased to 430 at week 2. EI decreased in all locations from week 4 (). Compared to EI measured at baseline, EI at week 4 were decreased by 31, 27, 13, and 25 units on the forehead, chin, right and left cheek respectively. Skin hydration and TEWL were also measured at four different anatomical areas. Although the skin hydration increased and TEWL decreased after exosome treatment, the changes were not statistically significant. (Supplementary Figures 2 and 3)
Figure 3. Changes in the erythema index (EI) were measured at four different anatomical locations on the face (forehead, left and right cheek, and chin). Erythema index (EI) decreased in all four areas after exosome treatment and decreased fastest on the forehead. *p < 0.05, **<0.01, ***<0.001, compared with baseline
Analysis of stratum corneum samples
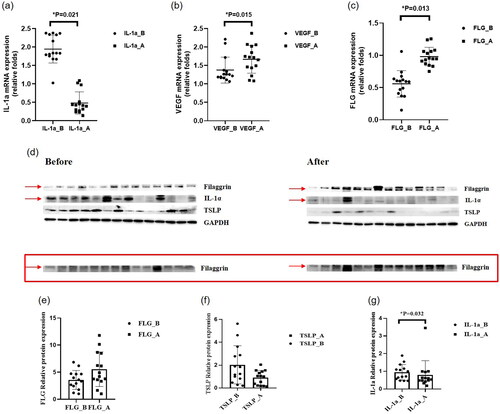
To determine the effect of ASCE on proinflammatory cytokine expression and epidermal proteins, we measured IL-1α, VEGF, TSLP, and FLG expression at mRNA and protein levels (). The mRNA expression of IL-1α decreased in SC samples collected at week 8 compared with the baseline. By contrast, the mRNA expression of FLG and VEGF increased increased at week 8 compared with the baseline. TSLP was not detected at the mRNA level. Regarding protein levels, ASCE upregulated FLG expression and downregulated both IL-1α and TSLP.
Figure 4. Results of the analysis of stratum corneum samples. (a) mRNA expression of interleukin-1α (IL-1α). The mRNA expression of IL-1α decreased in SC samples collected at week 8 compared with the baseline. (b) mRNA expression of vascular endothelial growth factor (VEGF). The mRNA expression of VEGF increased in SC samples collected at week 8 compared with the baseline. (c) mRNA expression of filaggrin (FLG). The mRNA expression of FLG increased in SC samples collected at week 8 compared with the baseline. (d) Western blot analysis. (e) Protein expression of filaggrin (FLG). The protein expression of FLG increased in SC samples collected at week 8 compared with the baseline. (f) Protein expression of human thymic stromal lymphopoietin (TSLP). The protein expression of TSLP decreased in SC samples collected at week 8 compared with the baseline. (g) Protein expression of interleukin-1α (IL-1α). The protein expression of IL-1α decreased in SC samples collected at week 8 compared with the baseline.
Discussion
The pathogenesis of DFR is still poorly understood, although several hypotheses exist. For example, it may represent a hypersensitivity reaction, a site-specific treatment failure, a seborrheic dermatitis-like reaction to Malassezia, a rosacea-like reaction associated with Demodex, allergic contact dermatitis, or a combination of multiple mechanisms (Citation26). The etiology of DFR appears to vary by individual because the histologic findings also vary and include psoriasiform dermatitis, spongiotic dermatitis with parakeratosis and neutrophil infiltration, or perifollicular and perivascular inflammation (Citation5,Citation27,Citation28). For the treatment of DFR, various topical formulations including corticosteroids, calcineurin inhibitors, and antifungal agents have been attempted, but were generally unsuccessful (Citation28–30). One potential reason for the variable treatment response could be the heterogeneous pathophysiology of DFR, meaning exosomes may be an ideal treatment option for DFR since they are highly immunomodulatory and have a pleiotropic effect on the target cells (Citation14,Citation18,Citation23,Citation31).
Our study investigated the possibility of applying a topical formulation containing ASCE to alleviate local skin inflammation seen in dupilumab-treated AD patients. Our results showed applying topical formulation containing ASCE quickly resolves DFR symptoms. The average IGA and CEA scores decreased from the second week of treatment. Notably, CEA decreased faster than IGA, which may be because erythema improves more than other DFR symptoms such as papulation or infiltration. The clinical improvement in erythema was also supported by the decrease in EI, which is a more objective measurement. EI decreased on the forehead from week 2, and the cheeks and chin from week 4. By contrast, patient satisfaction was highest in week 2 and slowly decreased, although a score of at least 3 was maintained. This may be because ASCE treatment resulted in an immediate improvement meaning patients were satisfied with the marked change after the first few treatment sessions but thereafter became less sensitive to further improvement in their skin.
As mentioned above, studies on the successful management of DFR are very limited. The relatively few case reports of the successful treatment of DFR include one where oral minocycline, topical tacrolimus, and brimonidine tartrate gel improved facial redness within a mean of 23 weeks (Citation5). In another case report, topical delgocitinib ointment improved DFR in one month (Citation32). The only reported clinical study on DFR treatment is a retrospective review evaluating the efficacy of oral itraconazole. The results showed 11 of 16 (69%) DFR patients had a post-treatment IGA result of clear or almost clear (0 or 1) one to six months later, with an average self-reported improvement of 52%. Our study showed similar results, where 12 of 20 (60%) patients achieved an IGA score of 1 (almost clear), and the average subjective satisfaction score was 3.6/572). More importantly, previous treatment methods such as oral itraconazole took at least a month or more to show any therapeutic effect. By contrast, the therapeutic effect of ASCE was noticeable after just two treatment sessions.
We also observed ASCE alleviated inflammation, upregulated skin barrier-related proteins, and balanced the Th1/Th2 immune response by reducing levels of TSLP. These findings were additionally supportive of the immunomodulatory and skin barrier-restoring functions of ASCE confirmed in previous murine studies. Previous studies have reported ASCE treatment relieved AD-like symptoms and reduced inflammatory cytokines (IL-4, IL-31, IL-23, and TNF-α), and also improved epidermal barrier functions by facilitating lamellar body formation and by de-repressing the synthesis of ceramides (Citation14,Citation18).
In this study, we found ASCE reduced inflammatory cytokine IL-1α. The IL-1 family cytokines initiate an inflammatory response. They have also been linked to the pathogenesis of AD as well as other inflammatory skin disorders (Citation33,Citation34). In particular, IL-1α (alarmin) is released from keratinocytes in any skin condition that involves damage to the epidermis (Citation35). Also, ASCE upregulated VEGF, which is a principal proangiogenic factor indicating ASCE can promote angiogenesis, which is consistent with many previous reports (Citation11,Citation19,Citation36–39). The repair of eczema lesions not only depends on the control of inflammation, but also depends on the repair and proliferation of keratinocytes and angiogenesis (Citation40). Last but not least, our results also demonstrated ASCE improves skin barrier function by upregulating FLG expression while suppressing TSLP (Citation41). FLG is a key epidermal protein that contributes to skin barrier function (Citation41,Citation42). Additionally, TSLP plays a key role in AD pathogenesis by triggering Th2 cell inflammation and by downregulating FLG expression (Citation43–46). Based on these results, we can presume that topical application of ASCE effectively alleviates DFR by suppressing cytokine-mediated inflammation and restoring the skin barrier function.
The major limitation of this study is the small number of patients and the lack of a control group. Therefore, we cannot completely rule out the possibility that DFR improved spontaneously. However, based on our clinical experience and previous reports on DFR, the possibility that DFR will improve spontaneously, especially within 2 weeks, seems very small. Thus, it can be assumed that the quick clinical improvement observed in this study is largely due to the effects of ASCE treatment. Furthermore, although there are limited numbers of studies on the management of DFR, we attempted to elaborate our conclusion by comparing the magnitude of improvement observed in our study to that observed in a previous study evaluating the efficacy of oral itraconazole. Despite this, in order to make a validated conclusion, further studies that uses vehicle control or that directly compares the effect of ASCE treatment with other treatment options such as oral itraconazole should be performed. In the future, larger, multi-institutional controlled studies are necessary to determine the extent of the efficacy of ASCE compared with other treatment options, as well as to establish a proper therapeutic approach, treatment dose, and regimen.
Conclusion
As the use of dupilumab for patients with AD gradually increases, the incidence of DFR will also increase. The successful management of DFR is important in increasing patient satisfaction with their dupilumab treatment and improving their quality of life. The results of our study demonstrated the potential of a topical formulation containing ASCE in the treatment of DFR. ASCE downregulated inflammation and increased skin barrier-related proteins and angiogenesis. Importantly, ASCE resulted in immediate clinical improvement of DFR, which has not been observed in other treatment methods. One of the most important advantages of ASCE is it can result in a therapeutic effect by topical application, which is important since DFR manifests as localized skin lesions. Thus, identifying an effective topical therapy may reduce the risks of adverse systemic effects and improve patient compliance.
Supplemental Material
Download PDF (291.5 KB)Acknowledgment
The authors wish to thank all of the dermatologists and collaborators who participated in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Additional information
Funding
References
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):1–8.
- de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin a or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):1083–1101.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303.
- Jang DH, Heo SJ, Jung HJ, et al. Retrospective study of dupilumab treatment for moderate to severe atopic dermatitis in Korea: efficacy and safety of dupilumab in real-world practice. J Clin Med. 2020;9(6):1982.
- Seok SH, An JH, Shin JU, et al. Facial redness in atopic dermatitis patients treated with dupilumab: a case series. Allergy Asthma Immunol Res. 2020;12(6):1063–1065.
- Soria A, Du-Thanh A, Seneschal J, et al. Development or exacerbation of head and neck dermatitis in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155(11):1312–1315.
- Lee DH, Ko HC, Na CH, et al. Real-World experience of Long-Term dupilumab treatment for atopic dermatitis in Korea. Ann Dermatol. 2022;34(2):157–160.
- Kozera E, Flora A, Stewart T, et al. Dupilumab-associated head and neck dermatitis resolves temporarily with itraconazole therapy and rapidly with transition to upadacitinib, with malassezia-specific immunoglobulin E levels mirroring clinical response. J Am Acad Dermatol. 2023;88(1):255–257.
- Ferrucci S, Angileri L, Tavecchio S, et al. Elevation of peripheral blood eosinophils during dupilumab treatment for atopic dermatitis is associated with baseline comorbidities and development of facial redness dermatitis and ocular surface disease. J Dermatolog Treat. 2022;33(5):2587–2592.
- Kee LT, Ng CY, Al-Masawa ME, et al. Extracellular vesicles in facial aesthetics: a review. Int J Mol Sci. 2022;23(12):6742.
- Weiliang Z, Lili G. Research advances in the application of adipose-derived stem cells derived exosomes in cutaneous wound healing. Ann Dermatol. 2021;33(4):309–317.
- Zhang B, Yin Y, Lai RC, et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–1244.
- Zhang B, Yeo RWY, Lai RC, et al. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy. 2018;20(5):687–696.
- Cho BS, Kim JO, Ha DH, et al. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9(1):187.
- Jang B, Chung H, Jung H, et al. Extracellular vesicles from Korean codium fragile and Sargassum fusiforme negatively regulate melanin synthesis. Mol Cells. 2021;44(10):736–745.
- Narauskaite D, Vydmantaite G, Rusteikaite J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14(8):811.
- Shen Y, Xu G, Huang H, et al. Sequential release of small extracellular vesicles from bilayered thiolated alginate/polyethylene glycol diacrylate hydrogels for scarless wound healing. ACS Nano. 2021;15(4):6352–6368.
- Shin KO, Ha DH, Kim JO, et al. Exosomes from human adipose Tissue-Derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680.
- Wang M, Zhao Y, Zhang Q. Human mesenchymal stem cell-derived exosomes accelerate wound healing of mice eczema. J Dermatolog Treat. 2022;33(3):1401–1405.
- Wang WM, Wu C, Jin HZ. Exosomes in chronic inflammatory skin diseases and skin tumors. Exp Dermatol. 2019;28(3):213–218.
- Xu P, Xin Y, Zhang Z, et al. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res Ther. 2020;11(1):264.
- Zhang Y, Yan J, Li Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate psoriasis-like skin inflammation. J Interferon Cytokine Res. 2022;42(1):8–18.
- Zhang B, Lai RC, Sim WK, et al. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int J Mol Sci. 2021;22(2):720.
- Park KY, Han HS, Park JW, et al. Exosomes derived from human adipose tissue-derived mesenchymal stem cells for the treatment of dupilumab-related facial redness in patients with atopic dermatitis: a report of two cases. J Cosmet Dermatol. 2022;21(2):844–849.
- Kwon HH, Yang SH, Lee J, et al. Combination treatment with human adipose tissue stem cell-derived exosomes and fractional CO2 laser for acne scars: a 12-week prospective, double-blind, randomized, split-face study. Acta Derm Venereol. 2020;100(18):adv00310.
- Ahn J, Lee DH, Na CH, et al. Facial erythema in patients with atopic dermatitis treated with dupilumab – a descriptive study of morphology and aetiology. Acad Dermatol Venereol. 2022;36(11):2140–2152.
- de Beer FSA, Bakker DS, Haeck I, et al. Dupilumab facial redness: positive effect of itraconazole. JAAD Case Rep. 2019;5(10):888–891.
- Nakanishi M, Tamagawa-Mineoka R, Arakawa Y, et al. Dupilumab-resistant facial erythema – dermoscopic, histological and clinical findings of three patients. Allergol Int. 2021;70(1):156–158.
- de Wijs LEM, Nguyen NT, Kunkeler ACM, et al. Clinical and histopathological characterization of paradoxical head and neck erythema in patients with atopic dermatitis treated with dupilumab: a case series. Br J Dermatol. 2020;183(4):745–749.
- Jo CE, Finstad A, Georgakopoulos JR, et al. Facial and neck erythema associated with dupilumab treatment: a systematic review. J Am Acad Dermatol. 2021;84(5):1339–1347.
- Moghadasi S, Elveny M, Rahman HS, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Jensen LE. Targeting the IL-1 family members in skin inflammation. Curr Opin Investig Drugs. 2010;11(11):1211–1220.
- Martin P, Goldstein JD, Mermoud L, et al. IL-1 family antagonists in mouse and human skin inflammation. Front Immunol. 2021;12:652846.
- Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107(6):2574–2579.
- Alkharsah KR. VEGF upregulation in viral infections and its possible therapeutic implications. Int J Mol Sci. 2018;19(6):1642.
- Hu L, Wang J, Zhou X, et al. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993.
- Zhang B, Wang M, Gong A, et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–2168.
- Zhang J, Chen C, Hu B, et al. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12(12):1472–1487.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743.
- Kim YJ, Choi MJ, Bak DH, et al. Topical administration of EGF suppresses immune response and protects skin barrier in DNCB-induced atopic dermatitis in NC/nga mice. Sci Rep. 2018;8(1):11895.
- Debinska A. New treatments for atopic dermatitis targeting skin barrier repair via the regulation of FLG expression. J Clin Med. 2021;10(11):2506.
- Kim JH, Bae HC, Ko NY, et al. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J Allergy Clin Immunol. 2015;136(1):205–208 e9.
- Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295.
- Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845–852.
- Ahn J, Choi Y, Simpson EL. Therapeutic new era for atopic dermatitis: part 1. Biologics. Ann Dermatol. 2021;33(1):1–10.