Abstract
Background
Using a three-pronged acne treatment approach—combining an antibiotic, antimicrobial agent, and retinoid—may provide greater efficacy than monad or dyad treatments. Herein are the dermal sensitization, irritation, safety, and tolerability results from phase 1 and 2 studies of fixed-dose clindamycin phosphate 1.2%/benzoyl peroxide (BPO) 3.1%/adapalene 0.15% (IDP-126) polymeric mesh gel.
Methods
Two phases 1, single-blind, vehicle-controlled dermal safety studies were conducted in healthy participants aged ≥18 years. One phase 2 (NCT03170388) double-blind, randomized, parallel-group, and vehicle-controlled study was conducted over 12 weeks in participants aged ≥9 years with moderate-to-severe acne.
Results
A total of 1,020 participants (IDP-126 gel, vehicle, or 1 of the 3 dyad gels [phase 2 only]) were included across the 3 studies (safety populations: n = 1,004). In the phase 1 studies, IDP-126 had no confirmed sensitization or contact dermatitis. IDP-126 (deemed “moderately irritating”) was significantly less irritating than commercially available BPO 2.5%/adapalene 0.3% gel.
Conclusions
The results from these three studies show that the triple-combination IDP-126 had a positive safety profile and was well tolerated in healthy participants and those with moderate-to-severe acne
Introduction
Acne vulgaris is one of the most common dermatologic disorders, affecting approximately 85% of adolescents and an increasing number of adults in the US (Citation1,Citation2). The pathogenesis of acne is a multifactorial process that comprises increased inflammation and sebum production, follicular proliferation of Cutibacterium acnes (formerly Propionibacterium acnes), and abnormal keratinization (Citation1, Citation3). As such, combination therapies are recommended in the US for most patients with acne (Citation1). Targeting multiple pathogenic pathways with combination treatment may provide better efficacy than a single treatment (Citation1) and a fixed-dose product may improve patient adherence by reducing treatment complexity compared to multiple concurrent treatments (Citation4). According to the most recent treatment guidelines developed by the American Academy of Dermatology, the recommended first-line treatments are topical benzoyl peroxide (BPO) or retinoids (e.g., tretinoin, adapalene, trifarotene, or tazarotene) as monotherapy, or in combination with each other and/or an antibiotic (Citation1). However, cutaneous irritation or dermatitis (either irritant [occurs rapidly post-contact] or allergic [a less common, delayed immune-mediated response]) may limit their use (Citation5–7), decrease treatment adherence, and/or lead to drug discontinuation (Citation8,Citation9).
Clindamycin phosphate 1.2%/BPO 3.1%/adapalene 0.15% (IDP-126) polymeric mesh gel is the first fixed-dose, triple combination treatment in development that addresses the major pathophysiological abnormalities of acne. IDP-126 was developed to provide a safe and effective topical acne treatment that targets three of the four acne pathogenic pathways, with a low irritability profile. This pH-balanced aqueous polymeric dispersion gel contains micronized BPO and adapalene. Polymeric mesh gel technology allows the suspended, micronized ingredients to be better distributed on the skin surface and may provide more efficient penetration of the pilosebaceous unit (Citation10). The formulation contains no preservatives, surfactants, alcohol, or occlusive agents, but does contain moisturizing ingredients, such as a low concentration of propylene glycol (a hydrating humectant), which may help reduce retinoid- or BPO-associated irritation (Citation11–13). In addition, combining topical BPO with topical clindamycin reduces the development of resistant strains of bacteria and may improve efficacy (Citation14–16), and monotherapy with topical antibiotics is not recommended per the treatment guidelines (Citation1). In a phase 2 study of participants with moderate-to-severe acne, once-daily IDP-126 demonstrated superior efficacy versus vehicle gel and three dyad component gels (BPO/adapalene, clindamycin phosphate/BPO, and clindamycin phosphate/adapalene), with 12-week treatment success rates of 52.5%, and good tolerability (Citation17). In the two identically designed, 12-week phase 3 pivotal trials, about half of the participants with moderate-to-severe acne treated once daily with IDP-126 achieved treatment success by week 12 versus less than one quarter with vehicle gel (p < 0.01, both). Furthermore, over 70% reductions in inflammatory and noninflammatory lesions were observed with IDP-126, and treatment was safe and well-tolerated (Citation18).
The objective of this article is to provide an overview of dermal sensitization, safety, and tolerability from phase 1 and 2 clinical studies of IDP-126 in healthy participants and those with moderate-to-severe acne.
Methods
Study designs and participants
Two phase 1, single-center (United States), evaluator-blinded, within-participant and vehicle-controlled dermal safety studies (repeat insult patch test [RIPT] study and cumulative irritation patch test [CIPT] study) were performed. RIPT studies determine the potential of a topical treatment to induce sensitization (allergic potential) through repeated applications; the methods for the study discussed herein are based on the modified Draize procedure (Citation7,Citation19). CIPT studies evaluate a topical treatment’s irritancy potential as a result of direct damage to the epidermal cells (without involvement of allergic or immunologic mechanism) via repeated applications (Citation7,Citation20); the methods for the study discussed herein are based on the modified Berger procedure.
In both studies, healthy participants aged ≥18 years who were not using systemic or topical corticosteroids (within 3 weeks prior to study day 1), did not have visible skin disease at the potential patch site, did not have an allergy/sensitivity to the patch constituents, and did not have psoriasis or active atopic dermatitis/eczema were eligible. Participants had 2 × 2 cm semi-occlusive patches (Webril® patch pad, Medline Industries Inc, Northfield, IL) applied to the skin of the upper back (infrascapular area).
In the RIPT study, ranging from 6 to 12 weeks in duration, each participant received a set of 3 patches. Each patch was coated with 0.2 ml of 1 of 3 treatments: clindamycin phosphate 1.2%/BPO 3.1%/adapalene 0.15% (IDP-126) gel, vehicle gel, and saline 0.9% solution (control). IDP-126 gel and vehicle gel were manufactured by Bausch Health Companies Inc. Participants were randomized to have the patches placed on either the right or left side of the back during the induction phase and on the opposite side during the challenge phase (). During the induction phase, patches for each treatment were placed on the same site for 48–72 (±6) h 3 times per week for 3 consecutive weeks (9 applications per treatment), with evaluations following patch removal. A rest period of 10–14 days followed the induction phase during which no patches were applied. In the challenge phase, patches for each treatment were placed on naïve skin sites for 48 ± 5 h with evaluations at 15 ± 10 min and 24 ± 6, 48 ± 6, and 72 ± 6 h following patch removal (1 application per treatment). If signs suggesting contact sensitization were observed for any treatment during the challenge phase, this phase was repeated for that treatment (re-challenge) approximately 4 weeks after the completion of the challenge phase. Induction and challenge phases occurred over a total of 6–7 weeks, with participants receiving a total of 10 patches per treatment. If re-challenge was required, then the study period occurred over a total of 10–12 weeks with an additional 11 patch applications ().
Figure 1. Study designs for the phase 1 RIPT (A) and CIPT (B) studies. RIPT study: determines the potential of a topical treatment to induce sensitization (allergic potential) via repeated applications over 6–12 weeks. CIPT study: evaluates a topical treatment’s irritancy potential as a result of direct damage to the epidermal cells (without involvement of allergic or immunologic mechanism) via repeated applications over 21 days. aParticipants were only re-challenged to a study material if there were signs suggestive of contact sensitization observed at any challenge evaluations or at the investigator’s discretion; this occurred approximately 4 weeks after the completion of the Challenge phase. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; RIPT: repeat insult patch test; SLS: sodium lauryl sulfate; tiw: three times weekly.
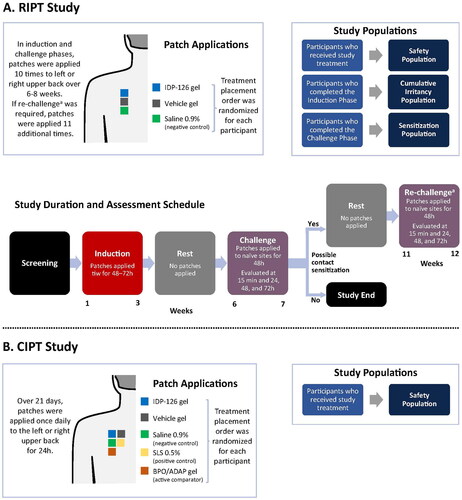
In the 21-day CIPT study, each participant received a set of 5 patches. Each patch was coated with 0.2 ml of 1 of 5 treatments: IDP-126 gel, vehicle gel, saline 0.9% solution (negative control), sodium lauryl sulfate 0.5% (SLS; positive control), and benzoyl peroxide (BPO) 2.5%/adapalene 0.3% gel (commercially available comparator [Epiduo® Forte] manufactured by Galderma Laboratories, LP). According to the randomization scheme, a set of 5 application sites was prepared. Placement on either the right or left side of the upper back was determined by the clinical staff based on skin clarity. Patches were applied every 24 ± 4 h over 21 consecutive days (). A trained, blinded investigator evaluated all test sites for symptoms following patch removal. In both phase 1 studies, skin sites were cleansed with tissue after patch removal and prior to the placement of new patches. Additionally, careful observation was performed to ensure any evidence of slight irritation or erythema was identified in participants with Fitzpatrick skin types IV-VI, as these changes may be more difficult to detect in patients with darker skin tones.
The 12-week phase 2 study was (NCT03170388) multicenter, double-blind, randomized, parallel-group, and vehicle controlled. Methods for this study have been published previously (Citation17). In brief, participants aged ≥9 years with moderate-to-severe acne were randomized (1:1:1:1:1) to receive IDP-126; vehicle gel; or one of the three component dyads formulated with the same active drug concentration and the same vehicle as IDP-126: BPO 3.1%/adapalene 0.15% gel; clindamycin phosphate 1.2%/BPO 3.1% gel; or clindamycin phosphate 1.2%/adapalene 0.15% gel. Treatments were applied to the face once daily for 12 weeks. Eligible participants had an Evaluator’s Global Severity Score (EGSS) rating of “moderate” (score = 3) or “severe” (score = 4) and had facial acne with inflammatory lesion counts of ≥30 to ≤100, noninflammatory lesion counts of ≥35 to ≤150, and ≤2 nodules. CeraVe® hydrating cleanser, CeraVe® moisturizing lotion (L’Oreal, New York, NY), and sunscreen were provided as needed for optimal moisturization/cleansing of the skin. Participants were enrolled at 31 sites in the United States and 4 in Canada.
All studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization, Good Clinical Practice Guidelines, and local regulations. All participants or their legal guardians provided written informed consent. Studies were approved by relevant institutional review boards or independent ethics committees at each study site.
Dermal sensitization and irritation assessments
In the phase 1 studies, cutaneous reaction assessments occurred at baseline and either 9 times during induction and 4 times during the challenge phase (RIPT), or 21 times post-baseline (CIPT). Any participants requiring a re-challenge (RIPT) had 4 additional assessments. Clinical grading of irritation consisted of a combination of letter and numerical grades (). All clinical grades were converted to scores of 0–3. Patch test scores at each evaluation were a summation of the letter and numerical grades (e.g., clinical grade of 3 C = 3 + 1 = sum score of 4), with a maximum possible score of 6. In the RIPT study, a sum score of ≥3 at the first or second reading in the induction phase would indicate pre-sensitization and the application of that treatment would be discontinued, with a score of 3 carried forward for the remainder of the induction phase. Similar reactions in subsequent readings would require a change in patch location or conditions for the remaining induction applications. A participant would be re-challenged to a certain treatment if any sign suggestive of contact sensitization (definite erythema with papules and/or edema) was observed at the 15 min or 24-, 48-, or 72-h assessments of the challenge phase. In the CIPT study, if a sum score ≥3 was observed for a specific treatment at patch removal, that treatment was terminated for the remainder of the study and a score of 3 was carried forward through any subsequent readings.
Table 1. Clinical Grading and Corresponding Numeric Scores (Phase 1 Studies).
Cutaneous safety and tolerability assessments
In all three studies, adverse events (AEs) and serious AEs (SAEs) were monitored throughout. Additionally, in the phase 2 study, investigator-assessed cutaneous safety (scaling, erythema, hypopigmentation, hyperpigmentation) and participant-assessed tolerability (itching, burning, stinging) were evaluated using a 4-point scale where 0 = none and 3 = severe.
Statistical analysis
In the phase 1 studies, the safety populations comprised all participants who received study treatment. In the RIPT study, the cumulative irritancy population comprised all participants who completed the induction phase. The sensitization population included all participants who completed the challenge phase. In the phase 2 study, the safety populations included all randomized participants who used the study drug at least once and had at least one post-baseline evaluation. The intent-to-treat population comprised all randomized participants who received the study drug.
In the phase 1 studies, mean cumulative and total irritation scores were calculated for the 9-day induction phase (RIPT) and days 2–22 (CIPT). Mean cumulative irritation score was calculated as the average score for all assessments and mean total irritation score was calculated as the sum of scores. To determine the irritation classification of each treatment in the CIPT study, a normalized total score for each patch was calculated by multiplying the mean total irritation score by a factor of 10 (scores: 0–49 = no significant irritation, 50–199 = slightly irritating, 200–449 = moderately irritating, and 450–630 = highly irritating) (Citation20). In both studies, irritation scores were analyzed pairwise using Fisher’s protected least significant differences in the context of a two-way analysis of variance, which included effects of participant and treatment, without interaction. Significant pairwise differences were tested at the 5% level. No imputations were made for missing data. All data were analyzed using SAS® software (v 9.2 or higher). In all three studies, demographic and safety data were summarized descriptively, and AEs were classified using the Medical Dictionary for Regulatory Activities (MedDRA) terminology.
Results
Participant disposition and demographics
Across the 3 studies, a total of 1,020 participants were randomized to clindamycin phosphate 1.2%/BPO 3.1%/adapalene 0.15% (IDP-126) gel, vehicle gel, or 1 of the 3 dyad gels (phase 2 only), and 1,004 participants were included in the safety populations. Participants in the phase 1 studies were older (mean age 55 years) and over two-thirds were Black, whereas those in phase 2 were generally younger (mean age 19 years) and approximately two-thirds were White (). In all studies, most participants were non-Hispanic/Latino.
Table 2. Participant Demographics and Baseline Characteristics (Safety Populations).
In the 6–12-week repeat insult patch test (RIPT) study, all 234 randomized participants were included in the safety population; of these, 209 (89.3%) comprised the cumulative irritancy population, and 210 (89.7%) completed the induction phase and received the challenge phase applications. A total of 206 participants (88.0%) completed the study and were included in the sensitization population. In the 21-day cumulative insult patch test (CIPT) study, 45 (100%) participants were included in the safety population, 44 (97.8%) were included in the irritation analysis, and 42 (93.3%) completed the study.
Safety
Dermal sensitization and irritation
A total of 5/206 (2.4%) participants in the RIPT study had suspected sensitization (4 to IDP-126 and 1 to vehicle) and received re-challenge phase application. Of these participants, 2 were deemed to have evidence suggestive of allergic sensitization (sum score of ≥3) to IDP-126 (1 borderline and 1 strongly suggestive); no participants, however, were confirmed by the investigator to have sensitization. IDP-126 gel, vehicle gel, and saline 0.9% were all classified as not having clinically significant irritation, though mean cumulative and total irritation scores were higher with IDP-126 versus vehicle or saline (p < 0.001, both; ).
Figure 2. Mean cumulative irritation scores (RIPT cumulative irritancy population; CIPT safety population). ***p < 0.001 vs IDP-126 gel. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; RIPT: repeat insult patch test; SD: standard deviation; SLS: sodium lauryl sulfate.
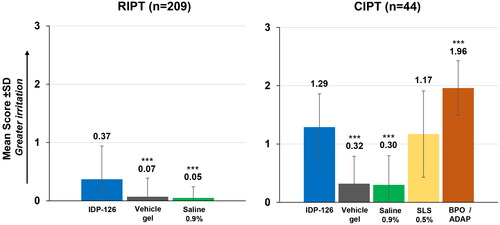
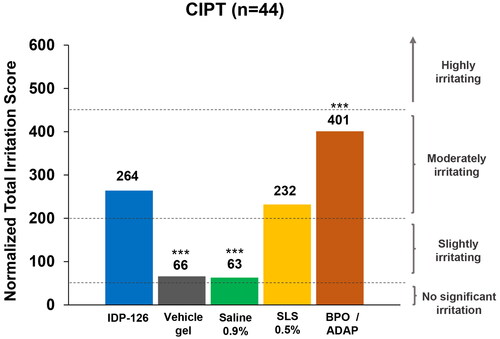
In the CIPT study, similar trends in mean and total irritation scores were observed (). Scores in the CIPT study were higher than those in the RIPT study due to the longer treatment duration. As expected, based on normalized total irritation scores (ranging from 0 = no significant irritation to 630 = highly irritating), IDP-126 was deemed “moderately irritating” with a score of 264 (200–449 = moderately irritating; ). Sodium lauryl sulfate (SLS) 0.5% was also “moderately irritating” (232), and vehicle and saline were “slightly irritating” (66 and 63, respectively). The highest irritation score was observed for BPO 2.5%/adapalene 0.3% gel, which was “moderately irritating” (401). IDP-126 had a statistically significantly higher irritation score than vehicle and saline (p < 0.001, both), but a significantly lower irritation score versus benzoyl peroxide (BPO)/adapalene (264 vs 401; p < 0.001; ).
Figure 3. Normalized total irritation scores (CIPT safety population). ***p < 0.001 vs IDP-126 gel. Normalized irritation scoring: 0–49 = no significant irritation; 50–199 = slightly irritating; 200–449 = moderately irritating; 450–630 = highly irritating. BPO/ADAP: benzoyl peroxide 2.5%/adapalene 0.3% gel; CIPT: cumulative irritancy patch test; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; SLS: sodium lauryl sulfate.
Adverse events
In the RIPT study, 4 (1.7%) participants experienced 4 treatment-emergent adverse events (TEAEs; ). None were considered treatment-related; 3 of these participants discontinued due to AEs (2 for COVID-19 and 1 for a vaccination-related adverse reaction). One participant experienced a severe AE of death during hospitalization for suspected COVID-19. In the CIPT study, 1 (2.2%) participant experienced a serious TEAE (pelvic fracture) which was moderate in severity, led to study/drug discontinuation and was deemed unrelated to treatment (). No participants experienced contact dermatitis. None of the participants in either study discontinued patch applications due to irritation.
Table 3. Treatment-Emergent Adverse Events (Safety Populations).
The safety results from the phase 2 study have previously been published (Citation17). Briefly, 36.2% of IDP-126-treated participants experienced TEAEs, similar to the BPO/adapalene-treated group (35.6%; ). A total of 4 participants reported any serious AEs (1 with IDP-126 and 3 with clindamycin/adapalene), none of which were deemed related to treatment. The majority of TEAEs were mild-to-moderate in severity. More participants discontinued the study or study drug with BPO/adapalene (5.5%) than with IDP-126 (2.8%), clindamycin/BPO (0%), clindamycin/adapalene (2.0%), or vehicle (1.4%; ).
The most commonly reported TEAEs were application site pain, dryness, and exfoliation (). In terms of dermatitis- and irritation-related AEs, 5 participants in the phase 2 study experienced dermatitis/contact dermatitis/atopic dermatitis (BPO/adapalene [2]; IDP-126 [1]; clindamycin/BPO [1]; clindamycin/adapalene [1]); 1 case was considered related to treatment (contact dermatitis: BPO/adapalene). Application site dermatitis was reported in 8 participants (IDP-126 [3]; BPO/adapalene [3]; clindamycin/adapalene [2]) and was deemed unrelated to treatment in 1 case (IDP-126). Application site irritation occurred in 13 participants (BPO/adapalene [5]; clindamycin/adapalene [4]; IDP-126 [3]; clindamycin/BPO [1]) and was considered unrelated in 2 cases (BPO/adapalene [1] and clindamycin/adapalene [1]). All cases were mild-to-moderate in severity.
Cutaneous safety and tolerability
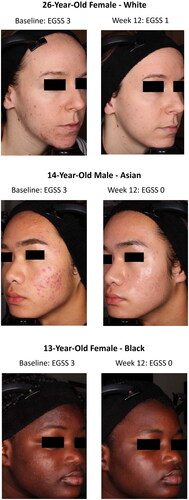
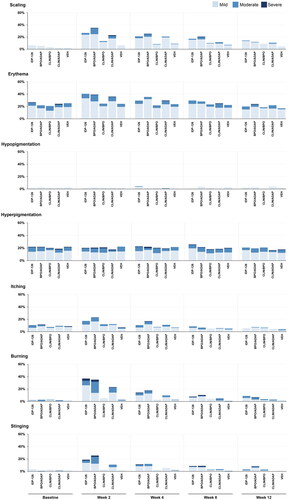
As previously reported for the phase 2 study (Citation17), there were transient increases in severity from baseline at weeks 2 or 4 across all active treatments on several cutaneous safety and tolerability assessments (). The highest rates of severe events at any timepoint were reported for hyperpigmentation (BPO/adapalene), stinging (BPO/adapalene), and burning (BPO/adapalene, IDP-126). With IDP-126, there were no reports of severe scaling or itching at any timepoint. For erythema and hyperpigmentation, <1% of IDP-126-treated participants were severe at baseline, which decreased to 0% by week 12 (). Less than 6% of participants in any treatment group experienced a treatment-emergent severe (Grade 3) rating on any cutaneous safety or tolerability assessment during the study (data not shown; see Stein Gold et al. 2022 (Citation17)). Photographs from participants treated with IDP-126 are shown in .
Figure 4. Cutaneous safety and tolerability by visit (Phase 2 safety population). Data for “none” are not shown. No imputation of missing data. Investigator-assessed cutaneous safety (scaling, erythema, hypopigmentation, hyperpigmentation) and participant-assessed tolerability (itching, burning, stinging) were evaluated using a 4-point scale where 0 = none and 3 = severe. ADAP: adapalene 0.15%; BPO: benzoyl peroxide 3.1%; CLIN: clindamycin phosphate 1.2%; IDP-126: clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/adapalene 0.15% gel; VEH: vehicle gel. Baseline N values: IDP-126 gel, n = 141; BPO/ADAP gel, n = 146; CLIN/BPO gel, n = 144; CLIN/ADAP gel, n = 148; VEH gel, n = 146.
Discussion
In addition to the efficacy demonstrated in clinical trials (Citation17, Citation21), the first fixed-dose, triple combination, clindamycin phosphate 1.2%/benzoyl peroxide (BPO) 3.1%/adapalene 0.15% (IDP-126) polymeric mesh gel in development for acne has shown a positive safety profile. IDP-126 was well tolerated under the exaggerated use conditions in the phase 1 repeat insult patch test (RIPT) and cumulative insult patch test (CIPT) studies as well as in the normal once-daily use conditions of the phase 2 clinical trial.
BPO and retinoids are associated with local cutaneous irritation, dryness, scaling, peeling, and/or erythema (i.e., contact dermatitis) (Citation5,Citation22). Such adverse effects may reduce patient compliance (Citation8,Citation9). Furthermore, formulations that are greasy, sticky, or gritty may be unpleasant for patients to use and could reduce treatment adherence (Citation10,Citation23). The IDP-126 aqueous gel is a pH-balanced polymeric dispersion formulation containing both hydrating and non-greasy moisturizing ingredients, which may help reduce the irritation that has been reported with retinoid and BPO use (Citation10,Citation12,Citation13). Additionally, ingredients such as alcohol, surfactants, occlusive agents, or preservatives—some of which are associated with irritation, dryness, and or/dermatitis (Citation24–26)—are not included.
In the present within-participant phase 1 studies of IDP-126, there was no observed sensitization, and the overall irritation potential was moderate and not clinically significant. In the RIPT study, there were no cases of investigator-confirmed sensitization with IDP-126. This low sensitization potential mirrors what has been observed in the literature for individual topical drugs (i.e., retinoids/adapalene, BPO, and clindamycin) (Citation5,Citation27). In terms of the irritation potential, IDP-126 was determined to have “no clinically significant irritation” (RIPT study) and was deemed “moderately irritating” (CIPT study). These results were expected and are consistent with BPO (Citation5) and topical retinoids (Citation22). Although the mean irritation score for IDP-126 was similar to the sodium lauryl sulfate (SLS) positive control in the CIPT study, this may be a result of the exaggerated dosing conditions. Of note, IDP-126 was significantly less irritating compared with the branded, commercially available BPO 2.5%/adapalene 0.3% gel. While both treatments had normalized irritation scores that fell within the “moderately irritating” range (defined as scores from 200–449), IDP-126 had a significantly lower score than BPO/adapalene (264 vs 401).
To put these results in context with other commercially available topical combination acne treatments, the mean cumulative and normalized total irritancy scores for IDP-126 (1.29; 264) were lower than those reported in other CIPT studies of dyads containing BPO and a retinoid or clindamycin: BPO 5%/clindamycin 1% gel and BPO 5%/clindamycin phosphate 1.2% gel (1.39, 1.36; 293, 286 (Citation28)) and BPO 3%/tretinoin 0.1% cream (1.59; 333 (Citation29)). Direct comparisons, however, cannot be made as these were not head-to-head studies. Also, variations in the study populations, methods, or topical formulations may have resulted in differences in irritancy scores.
When applied to the face once daily under unoccluded conditions, IDP-126 was well tolerated. In the overall population of the phase 2 study, mean scores on cutaneous safety and tolerability measures were ≤0.6 (score of 1 = mild) for all treatments at all time points (Citation17). While tolerability can affect adherence (Citation4,Citation8), cutaneous irritation could also lead to the development, or exacerbation, of dyspigmentation (Citation30,Citation31). Dyspigmentation is an acne-related sequela that can persist long after the acne has resolved and may be more distressing to the patient than the acne itself (Citation30,Citation32). Patients with lighter skin types (Fitzpatrick I-III) are more likely to experience pink-to-red discoloration (post-inflammatory erythema) (Citation31), while those with darker skin types (IV-VI) are more likely to experience areas of darker discoloration (post-inflammatory hyperpigmentation [PIH]) (Citation33). In the present phase 2 study, the percentage of participants with any erythema or hyperpigmentation with IDP-126 decreased slightly from baseline to week 12. Less than 1% of IDP-126-treated participants had severe erythema or hyperpigmentation at baseline, which decreased to 0% by week 12. The BPO/adapalene dyad was the only treatment to have an increase in the percentage of participants experiencing severe erythema from baseline to week 12 (0% to 0.8%).
Interestingly, rates of severe participant-reported stinging and/or burning in the IDP-126 phase 2 study were highest with the BPO/adapalene dyad than with IDP-126 or the other two dyads containing clindamycin (clindamycin/adapalene, clindamycin/BPO) (Citation17). The inclusion of clindamycin in IDP-126 may have provided a moderating effect on the cutaneous tolerability and safety of adapalene or BPO through its multiple anti-inflammatory properties (Citation34). Indeed, a meta-analysis demonstrated lower odds of patient discontinuation or withdrawal due to adverse events (AEs) for clindamycin combined with BPO than for BPO/adapalene combinations or BPO alone (Citation35).
One concern with combining multiple active ingredients to treat acne is a possible increase in the number of AEs and/or reduction in tolerability. In all three of the present studies, IDP-126 was not only well tolerated, but also had a positive safety profile. Treatment-emergent AE (TEAE) rates with IDP-126 were 1.7% to 2.2% in the phase 1 studies (none related to treatment) and 35.6% in the phase 2 study (19.9% related). Most were of mild-to-moderate severity and no serious AEs were related to treatment. The IDP-126 TEAE rates in the phase 2 study (36.2%) were similar to those observed in other clinical trials of topical dyads containing a combination of BPO, clindamycin, or adapalene (range of AEs: 23.0% to 47.3%) (Citation14,Citation36–40). The rate of discontinuations due to AEs with IDP-126 (2.8%) was also similar to the dyads in other clinical trials (range: 0% to 2.7%) (Citation14,Citation36–40).
There are several limitations of the present studies. The generalizability of the phase 1 results may be limited as these participants were healthy and tended to be older (mean age: 55 years) than participants in the phase 2 study (19 years) who had moderate-to-severe acne. Additionally, the generalizability of the phase 2 study results is limited as the study was designed to evaluate 12 weeks of treatment in participants with moderate-to-severe acne. Any effect of age, race, or genetics on irritation/dermatitis was beyond the scope of these analyses.
Conclusion
The fixed-dose, triple-combination clindamycin phosphate 1.2%/BPO 3.1%/adapalene 0.15% (IDP-126) polymeric mesh gel had moderate irritancy and had no confirmed sensitization/contact dermatitis under exaggerated dosing conditions. Additionally, IDP-126 was significantly less irritating than the commercially available Epiduo Forte (BPO 2.5%/adapalene 0.3%) gel. Overall, IDP-126 has a positive safety profile and is well tolerated in healthy patients and those with moderate-to-severe acne.
Acknowledgements
Medical writing support was provided by Lynn M. Anderson, Ph.D. and Jacqueline Benjamin, Ph.D. of Prescott Medical Communications Group (Chicago, IL) with financial support from Ortho Dermatologics. Ortho Dermatologics is a division of Bausch Health US, LLC.
Disclosure statement
Zoe D. Draelos received research funding from Ortho Dermatologics. Emil A. Tanghetti has served as a speaker for Novartis, Ortho Dermatologics, Sun Pharma, Lilly, Galderma, AbbVie, and Dermira; served as a consultant/clinical studies for Hologic, Ortho Dermatologics, and Galderma; and is a stockholder for Accure. Leon H. Kircik has acted as an investigator, advisor, speaker, and consultant for Ortho Dermatologics. Neal Bhatia has served as advisor, consultant, and investigator for AbbVie, Almirall, Biofrontera, BI, Brickell, BMS, EPI Health, Ferndale, Galderma, InCyte, ISDIN, J&J, LaRoche-Posay, LEO Pharma, Ortho Dermatologics, Regeneron, Sanofi, SunPharma, Verrica, and Vyne. Joshua A. Zeichner has served as advisor, consultant, or speaker for AbbVie, Allergan, Dermavant, Dermira, EPI Health, Galderma, Incyte, Johnson and Johnson, L’Oreal, Ortho Dermatologics, Pfizer, Procter and Gamble, Regeneron, Sun Pharma, UCB, Unilever, and Vyne. Jeffrey L. Sugarman is a consultant for Ortho Dermatologics, Bausch Health, Regeneron, Sanofi, Verrica, and Pfizer. Linda Stein Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly.
Data availability statement
Data available upon request.
Additional information
Funding
References
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):1–10.
- Skroza N, Tolino E, Mambrin A, et al. Adult acne versus adolescent acne: a retrospective study of 1,167 patients. J Clin Aesthet Dermatol. 2018;11(1):21–25.
- Gollnick HP, Zouboulis CC, Akamatsu H, et al. Pathogenesis and pathogenesis related treatment of acne. J Dermatol. 1991;18(9):489–499.
- Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029.
- Foti C, Romita P, Borghi A, et al. Contact dermatitis to topical acne drugs: a review of the literature. Dermatol Ther. 2015;28(5):323–329.
- Leyden J, Stein-Gold L, Weiss J. Why topical retinoids are mainstay of therapy for acne. Dermatol Ther. 2017;7(3):293–304.
- Nguyen HL, Yiannias JA. Contact dermatitis to medications and skin products. Clin Rev Allergy Immunol. 2019;56(1):41–59.
- Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456.
- Sevimli Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992.
- Kircik LH, Draelos ZD, Berson DS. Polymeric emulsion technology applied to tretinoin. J Drugs Dermatol. 2019;18(4):S148–S154.
- Baldwin H, Webster G, Stein Gold L, et al. 50 Years of topical retinoids for acne: evolution of treatment. Am J Clin Dermatol. 2021;22(3):315–327.
- Hoffman LK, Bhatia N, Zeichner J, et al. Topical vehicle formulations in the treatment of acne. J Drugs Dermatol. 2018;17(6):S6–S10.
- Tanghetti EA, Draelos ZD, Grimes P. Moisturizer use enhances facial tolerability of tazarotene 0.1% cream without compromising efficacy in patients with acne vulgaris. Poster presented at: Fall Clinical Dermatology, Las Vegas, NV, October 2008.
- Leyden JJ, Berger RS, Dunlap FE, et al. Comparison of the efficacy and safety of a combination topical gel formulation of benzoyl peroxide and clindamycin with benzoyl peroxide, clindamycin and vehicle gel in the treatments of acne vulgaris. Am J Clin Dermatol. 2001;2(1):33–39.
- Cunliffe WJ, Holland KT, Bojar R, et al. A randomized, double-blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther. 2002;24(7):1117–1133.
- Leyden JJ, Wortzman M, Baldwin EK. Antibiotic-resistant propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis. 2008;82(6):417–421.
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase 2 study of the first triple-combination drug. Am J Clin Dermatol. 2022;23(1):93–104.
- Stein Gold L, Kircik LH, Tanghetti EA. 32970: Efficacy and safety of a fixed-dose clindamycin 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: randomized phase 2 and phase 3 studies of the first triple-combination drug. JAAD. 2022;87(3).
- Jordan WP. 24-, 48-, and 48/48-hour patch tests. Contact Derm. 1980;6(2):151–152.
- Berger RS, James PB. A reappraisal of the 21-day cumulative irritation test in man. J Toxicol, Cutan Ocul Toxicol. 1982;1(2):109–115.
- Stein Gold L, Kircik LH, Tanghetti EA, et al. Efficacy and safety of a fixed-dose clindamycin 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: randomized phase 2 and phase 3 studies of the first triple-combination drug. Poster presented at: American Academy of Dermatology Annual Meeting, Boston, MA, March 2022.
- Culp L, Moradi Tuchayi S, Alinia H, et al. Tolerability of topical retinoids: are there clinically meaningful differences among topical retinoids? J Cutan Med Surg. 2015;19(6):530–538.
- Tanghetti EA, Stein Gold L, Del Rosso JQ, et al. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J Dermatolog Treat. 2021;32(4):391–398.
- Chou M, Mikhaylov D, Strugar TL. Common allergens present in personal care products: identification, diagnosis, and management. Semin Cutan Med Surg. 2018;37(4):254–262.
- Goosens C. Cosmetic contact allergens. Cosmetics. 2016;3(1):5.
- Barnes TM, Mijaljica D, Townley JP, et al. Vehicles for drug delivery and cosmetic moisturizers: review and comparison. Pharmaceutics. 2021;13(12):2012.
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8(4):377–381.
- Dosik JS, Vamvakias G. Comparative irritation potential of two combination acne products: a randomized controlled, subject- and evaluator-blind, comparative study in healthy volunteers. Am J Clin Dermatol. 2008;9(5):313–317.
- NDA-214902: US Food and Drug Administration; 2018. [cited 2022 November 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214902Orig1s000MultidisciplineR.pdf.
- Alexis AF, Harper JC, Stein Gold LF, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(3S):S71–S73.
- Bae-Harboe YS, Graber EM. Easy as pie (postinflammatory erythema). J Clin Aesthet Dermatol. 2013;6(9):46–47.
- Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7(7):19–31.
- Layton A, Alexis A, Baldwin H, et al. Identifying gaps and providing recommendations to address shortcomings in the investigation of acne sequelae by the personalising acne: consensus of experts panel. JAAD Int. 2021;5:41–48.
- Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85(1):15–24.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild-to-moderate acne vulgaris: systematic review and network meta-analysis. Br J Dermatol. 2021;185(3):512–525.
- Tschen EH, Katz HI, Jones TM, et al. A combination benzoyl peroxide and clindamycin topical gel compared with benzoyl peroxide, clindamycin phosphate, and vehicle in the treatment of acne vulgaris. Cutis. 2001;67(2):165–169.
- Thiboutot DM, Weiss J, Bucko A, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57(5):791–799.
- NDA-207917: US Food and Drug Administration; 2015. [cited 2022 November 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207917Orig1s000MedR.pdf.
- NDA-050819: US Food and Drug Administration; 2008. [cited 2022 November 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/050819s000_MedR.pdf.
- NDA-022320: US Food and Drug Administration; 2008. [cited 2022 November 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022320s000_MedR.pdf.