Abstract
The management of plaque psoriasis that affects difficult-to-treat areas can be challenging. Biologics have become the treatment of choice for moderate-to-severe plaque psoriasis. However, there are limited data on their efficacy in difficult-to-treat sites (including scalp, palms/soles, nails and genitalia). We conducted a 52-week retrospective study to evaluate the effectiveness of risankizumab in 202 patients with moderate-to-severe involvement of at least one difficult-to-treat area. One hundred and sixty-five patients had scalp psoriasis, 21 had involvement of palms or soles, 72 were affected by genital psoriasis, and 50 patients reported the involvement of the fingernails. After one year of treatment, 97.58% of patients with scalp involvement, 95.28% of patients with palmoplantar psoriasis, 100% of patients with genital psoriasis and 82% of patients with nail involvement achieved a site-specific Physician’s Global Assessment of 0 or 1 (clear or almost clear). No serious adverse events were observed during the study. Our study supports the effectiveness of risankizumab in plaque psoriasis involving difficult-to-treat sites.
Introduction
Plaque psoriasis most commonly occurs on the elbows, knees, and lumbosacral region, but it can affect any body surface (Citation1). Plaque psoriasis can also affect areas such as the scalp/face (43–65%), nails (23–60%), palms/soles (12–26%), and genitalia (14–43%), which have been described as difficult-to-treat areas (Citation2–5). Treatment of these patients can be challenging due to inadequate response to topical corticosteroids and vitamin D derivatives (Citation5). Cyclosporine and methotrexate are options, however, long-term treatment with cyclosporin causes renal dysfunction, hypertension, and blood count abnormalities, and methotrexate is associated with hepatic and hematological toxicities (Citation6).
Biologics offer safer alternatives, but there are limited data regarding their efficacy in patients with difficult-to-treat sites. Moreover, patients with limited disease extent are usually excluded from phase III clinical trials, despite the severe involvement of these areas (Citation4). Risankizumab is a highly effective and very safe inhibitor of interleukin (IL)-23 evaluated in clinical trials and real-world experiences (Citation7,Citation8). However, there is still a paucity of data regarding the use of this drug in psoriatic patients with moderate-to-severe involvement of the scalp, nails, palms/soles, and genitalia. The purpose of this study was to assess real-world risankizumab efficacy and safety in patients with psoriasis in these areas.
Methods
We conducted a 52-week retrospective study from two Dermatology Units to evaluate the effectiveness of risankizumab in patients with moderate-to-severe involvement of at least one difficult-to-treat area (defined by a site-specific Physician’s Global Assessment ≥ 3). Risankizumab was administered according to the Summary of Product Characteristics (Citation9). Scalp-specific Physician’s Global Assessment (sc-PGA), palmoplantar PGA (ppPGA), static Physician’s Global Assessment of Genitalia (sPGA-G), fingernail PGA (f-PGA), and Psoriasis Area and Severity Index (PASI) were recorded at each dermatological examination. Any adverse events (AEs) during the visits were also evaluated. The primary endpoints were the proportion of patients achieving a site-specific PGA of 0/1 (clear or almost clear) at weeks 16, 28, and 52.
Continuous data were reported as mean and standard deviation (SD), while categorical variables were presented as absolute numbers and percentages. The within-group comparison of mean-PGA (between baseline with weeks 16, 28, and 52) was performed by the Student’s t-test.
Results
We included 202 patients treated with risankizumab for at least 52 weeks (). One hundred and thirty-three were males (65.8%) with a mean age of 50.43 (SD 15.95). Our patients were affected by psoriasis for a mean of 17.27 years (SD 14.32). The mean body mass index (BMI) was 27.37 (SD 5.30), while 72 patients had at least one cardiometabolic comorbidity (arterial hypertension, obesity, type II diabetes mellitus, hypercholesterolemia, and cardiovascular diseases). Forty-six patients had previously received at least one biological drug (22.8%). Regarding the 165 patients with scalp involvement, sc-PGA of 0/1 was achieved in 86.7% (n = 143), 97.6% (n = 161), and 97.6% (n = 161) of them at weeks 16, 28, and 52, respectively (). Twenty-one patients were affected by palmoplantar psoriasis. After 16 weeks, 52% (n = 11) of them achieved a ppPGA of 0/1, with continuous improvement at weeks 28 (76%, n = 16) and 52 (95%, n = 20) (). Among the 72 patients with genital psoriasis, sPGA-G of 0/1 was observed in 85% (n = 61) at week 16, 93% (n = 67) at week 28, and 100% (n = 72) after one year (). Finally, 50 patients presented with nail psoriasis. A f-PGA of clear or almost clear was reached by 38% (n = 19) at week 16, 68% (n = 34) at week 28 and 82% (n = 41) at week 52 (). Mean PGA improved in each difficult-to-treat area. At baseline, the mean sc-PGA was 3.33 and decreased to 0.51 at week 15, 0.23 at week 28 and 0.16 after one year of treatment (). Mean pp-PGA decreased from a mean of 3.19 at baseline to 1.38 at week 16, 0.81 at week 28, and 0.43 at week 52 (). Mean sPGA-G decreased from a mean of 3.32 at baseline to 0.51, 0.25, and 0.14 at the same time points (). Mean f-PGA was 3.26 at baseline and decreased to 1.76 at week 16, 1.12 at week 28, and 0.70 after 52 weeks of treatment (). No significant safety findings were reported during the study, as no patient had to discontinue the drug because of treatment-emergent AEs or serious AEs.
Figure 1. Percentage of patients achieving sc-PGA (1a), pp-PGA (1b), sPGA-G (1c) and f-PGA (1d) of 0 or 1 (clear and almost clear) at weeks 16, 28 and 52.
Abbreviation: sc-PGA: scalp-specific Physician’s Global Assessment; pp-PGA: palmoplantar PGA sPGA-G: static Physician’s Global Assessment of Genitalia; f-PGA: fingernail PGA.
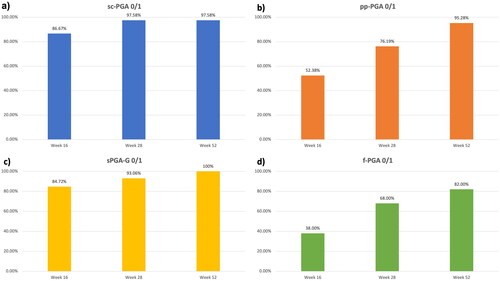
Figure 2. Mean site-specific PGA of our population at baseline, week 16, week 28 and week 52.
Abbreviation: sc-PGA: scalp-specific Physician’s Global Assessment; pp-PGA: palmoplantar PGA sPGA-G: static Physician’s Global Assessment of Genitalia; f-PGA: fingernail PGA; * p-value < 0.001.
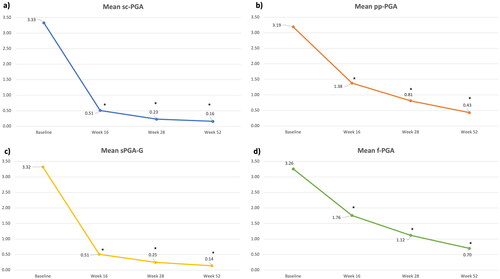
Table 1. Demographic characteristics and disease severity scores at baseline of our population.
Conclusions
Despite a large number of real-world studies and clinical trials on the efficacy of biologics in plaque psoriasis, few studies have focused on difficult-to-treat areas (Citation10,Citation11). Regarding IL-17 inhibitors, there have been clinical trials focused on difficult-to-treat sites: in particular, ixekizumab is effective for genital psoriasis (Citation12) and secukinumab for psoriasis involving the palms or soles (Citation13). Data from clinical trials regarding the role of anti-IL-23 drugs in treating these areas are currently limited. However, IL-23 inhibitors have comparable effectiveness in real-world experiences in patients with and without the involvement of difficult-to-treat areas (Citation14–17). In our study, risankizumab was rapidly effective, particularly in patients with scalp and genital psoriasis, which improved patient well-being.
Clinical Dermatology Unit - San Gallicano Dermatological Institute IRCCS, Rome, ItalyLuigi Gargiulo*, Luciano Ibba, Ruggero Cascio Ingurgio and Mario ValentiDermatology Unit, IRCCS Humanitas Research Hospital, Rozzano, MI, Italy Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy[email protected] PeruginiDermatology Unit, IRCCS Humanitas Research Hospital, Rozzano, MI, ItalyAlessia PacificoClinical Dermatology Unit - San Gallicano Dermatological Institute IRCCS, Rome, ItalyFabio S. MaramaoUOSD di Dermatologia, Fondazione Policlinico Tor Vergata, Università degli studi di Roma Tor VergataAntonio CostanzoDermatology Unit, IRCCS Humanitas Research Hospital, Rozzano, MI, ItalyDepartment of Biomedical Sciences, Humanitas University, Pieve Emanuele, ItalyAlessandra NarcisiDermatology Unit, IRCCS Humanitas Research Hospital, Rozzano, MI, Italy*Luigi Gargiulo and Diego Orsini contributed equally to this manuscript.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. The patient received risankizumab as in good clinical practice, in accordance with European guidelines. The patient and her parents had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores) and for the publication of clinical pictures. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Acknowledgements
None.
Disclosure statement
L. Gargiulo has been a consultant for Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data are available on request from the authors.
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–4.
- Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29(8):754–760.
- Chan CS, Van Voorhees AS, Lebwohl MG, et al. Treatment of severe scalp psoriasis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2009;60(6):962–971.
- Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589.
- Canal-García E, Bosch-Amate X, Belinchón I, et al. Nail psoriasis. Psoriasis ungueal. Actas Dermosifiliogr. 2022;113(5):481–490.
- Balak DMW, Gerdes S, Parodi A, et al. Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther . 2020;10(4):589–613.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in -to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661.
- Gargiulo L, Ibba L, Pavia G, et al. Real-life effectiveness and safety of Risankizumab in 131 patients affected by moderate-to-severe plaque psoriasis: a 52-week retrospective study. Dermatol Ther . 2022;12(10):2309–2324.
- 17. European Medicines Agency. Skyrizi (risankizumab): summary of product characteristics. 2019. [cited 2023, April 04]. http://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi.
- Ibba L, Gargiulo L, Alfano A, et al. Anti-IL-23 and anti-IL-17 drugs for the treatment of non-pustular palmoplantar psoriasis: a real-life retrospective study [published online ahead of print, 2023 Apr 3]. J Dermatolog Treat. 2023;34(1):2199108.
- Galluzzo M, Talamonti M, Cioni A, et al. Efficacy of Tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. JCM. 2022;11(9):2631. Published 2022 May 7.
- Guenther L, Potts Bleakman A, Weisman J, et al. Ixekizumab results in persistent clinical improvement in moderate-to-severe genital psoriasis during a 52 week, randomized, placebo-controlled, phase 3 clinical trial. Acta Derm Venereol. 2020;100(1):adv00006. Published 2020 Jan 7.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80.
- Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J Eur Acad Dermatol Venereol. 2023;37(1):93–103.
- Mastorino L, Susca S, Megna M, et al. Risankizumab shows high efficacy and maintenance in improvement of response until week 52. Dermatol Ther. 2022;35(5):e15378.
- Megna M, Tommasino N, Potestio L, et al. Real-world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an Italian 28-week retrospective study. J Dermatolog Treat. 2022;33(6):2813–2820.
- Gargiulo L, Ibba L, Malagoli P, et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 104-week multicenter retrospective study - IL PSO (ITALIAN LANDSCAPE PSORIASIS) [published online ahead of print, 2023 Jan 25]. Acad Dermatol Venereol. 2023;37(5):1017–1027.

