Abstract
Background
Patients with moderate-to-severe atopic dermatitis (AD) experience skin lesions and intense itch that substantially affect quality of life. Patients have choices among systemic AD treatments that offer varied benefit–risk profiles.
Objective
Measure patients’ willingness to trade off the risks and benefits of systemic treatments among individuals with a physician-confirmed diagnosis of moderate-to-severe AD.
Methods
Patients participated in a discrete choice experiment online survey with a series of choices between hypothetical AD treatments defined by six attributes reflecting benefits and risks of treatments (itch reduction, time until noticeable itch reduction, chance of clear or almost clear skin, risk of serious infection, risk of developing acne, and need for prescription topical steroids). Data were analyzed with a random parameters logit model to quantify preferences and the relative importance of attributes for treatment alternatives.
Results
Respondents (n = 200) placed the highest relative importance on itch reduction, speed of itch reduction, and skin clearance, and were generally willing to accept clinically relevant levels of risk of serious infection and acne in exchange for treatment benefits.
Conclusions
Patients with moderate-to-severe AD were willing to trade clinically relevant treatment risks for greater or more rapid itch reduction and skin clearance offered by systemic therapies.
Introduction
Atopic dermatitis (AD) affects approximately 16.5 million adults in the United States (US), with over one-third of cases classified as moderate to severe in intensity (Citation1). Patients with moderate-to-severe AD experience intense pruritus and skin pain that compromise sleep, work and school productivity, and performance of daily activities (Citation2,Citation3). These symptoms and activity limitations are associated with adverse psychosocial sequelae (e.g., anxiety, depression, and/or suicidal ideation) (Citation4) and diminished health-related quality of life (HRQoL) (Citation2,Citation3,Citation5).
Standard treatments for AD currently include anti-inflammatory topical therapies (e.g., topical corticosteroids, calcineurin inhibitors, phosphodiesterase-4 inhibitors, and Janus kinase inhibitors) and ultraviolet phototherapy. Systemic therapies are recommended for patients with more severe forms of AD if their symptoms are not adequately controlled with topical treatments or if patients’ HRQoL is severely affected by AD (Citation6,Citation7). In addition to immunosuppressants (e.g., corticosteroids, cyclosporine, azathioprine, methotrexate, and mycophenolate mofetil), multiple systemic therapies are currently available or in development to treat AD. These therapies include biologic interleukin inhibitors (e.g., dupilumab and tralokinumab) and small molecule oral Janus kinase inhibitors (e.g., baricitinib, abrocitinib, and upadacitinib) (Citation6,Citation8,Citation9). Highly effective, targeted therapies are needed, given the negative effects on HRQoL and medical care costs associated with AD (Citation4,Citation6).
Despite the widely appreciated impact of moderate-to-severe AD, some systemic approaches do not fully resolve pruritus or provide skin clearance (Citation10). Furthermore, some AD treatments are associated with greater risks of acne, nausea, conjunctivitis, facial redness, infection, malignancies, and other sequelae (Citation10–14). In real-world settings in patients with AD, the most common adverse event (AE) associated with upadacitinib was acne (consistent with findings in phase 3 studies) (Citation15), while rates of conjunctivitis and eosinophilia associated with dupilumab were higher in real-world settings than those reported in clinical trials (Citation16). Adverse events commonly associated with baricitinib treatment (acne, nasopharyngitis, headache, and elevated blood creatine phosphokinase levels) were also consistent between real-world settings and clinical trials (Citation17–19). Safety profiles associated with abrocitinib and tralokinumab in real-world settings are currently under investigation (Citation20–22). As AD treatment decisions dictate tradeoffs among efficacy, side effects, route of administration, and other factors, physicians should determine patients’ most-valued treatment attributes given the rapidly changing treatment landscape.
Discrete choice experiments (DCEs) enable quantification of treatment preferences and the tradeoffs that patients are willing to make between the benefits and risks of various interventions (e.g., better itch reduction in exchange for a higher risk of serious infection) (Citation23). These data provide insight into how patients value benefits and risks associated with different treatments. In AD, DCE studies have been limited to determining Spanish physicians’ treatment preferences (Citation24), Japanese patients’ preferences for injection-based AD therapies (Citation25), preferences for systemic AD approaches among patients in the US and United Kingdom (UK) (Citation26), and patients’ preferences for AD medications among patients in the UK, France, and Spain (Citation27). In addition to scant data regarding US patients’ preferences for AD treatments, no DCE study has enrolled patients with physician diagnosed moderate-to-severe AD. Given that patients experiencing AD symptoms are the most likely to be encountered in the clinic, knowledge of patients’ AD treatment preferences may guide conversations between patients and their physicians to develop individualized treatment plans.
With the availability of new treatments that offer improved efficacy with different safety profiles, physicians need to understand the benefit–risk tradeoffs preferred by patients. The primary objective of this cross-sectional, web-based DCE survey study was to explore preferences for a set of benefits and risks associated with systemic therapies among respondents with physician-confirmed moderate-to-severe AD who resided in the US.
Patients and methods
Study population
Respondents were recruited through physicians by Global Perspectives (Asturias, Spain), an international healthcare research organization. Respondents were required to be aged ≥18 years, a US resident, able to read and understand English, and have a physician-confirmed diagnosis of moderate or severe AD. Potential respondents were invited to participate via a standardized email linking them to an online study description; potential respondents also provided informed consent. The study was reviewed by the RTI International (Research Triangle Park, NC) institutional review board. All procedures followed the guidelines outlined in the Declaration of Helsinki and good clinical practice of the International Conference on Harmonization.
Survey instrument development and administration
A cross-sectional, web-based DCE survey was developed and administered to 200 respondents. DCE studies provide a quantitative measure of the tradeoffs respondents are willing to make among treatment attributes used in the study and have been widely used in health research (Citation23). The study was designed and conducted in accord with best practice guidelines for DCE studies (Citation28–30).
Respondents answered a series of DCE questions that offered a choice between pairs of hypothetical AD treatment profiles. Each hypothetical treatment profile consisted of an experimentally designed combination of attributes and levels. The attributes used to characterize the treatments were selected to align with the treatment-related benefits and risks associated with standard and recently developed systemic therapies for moderate-to-severe AD. The attributes included: ‘improvement in level of itch after 4 months of treatment’; ‘time until a noticeable itch reduction after treatment starts’; ‘separate from itch improvement, the chance of clear or almost clear skin after using the medicine for 4 months’; ‘annual risk of a serious infection from treatment’; ‘annual risk of developing acne from treatment’; and ‘need to use prescription topical steroids’. The selection of attributes and levels was based on research on patient preferences, clinical trial data, and studies of AD treatments. Supplemental Figure 1 presents an example DCE question for a respondent with baseline itch of 8 (the levels of the itch reduction attribute were based on the respondents’ report of their worst level of itch in the last seven days). The survey included plain-language descriptions of the attributes, questions about the respondents’ experience with AD and AD treatments, questions to confirm comprehension of key concepts, and questions regarding standard demographic items. The survey was evaluated in 14 individual web conference pretest interviews, and revisions were made to improve comprehension and readability based on the responses from the pretest interviews.
The experimental design for the DCE questions was generated using SAS statistical software (SAS version 9.4; SAS Institute Inc., Cary, NC) that employed a D-optimal algorithm to construct a fractional factorial experimental design. The final design contained 72 DCE choice questions, which were used to create eight blocks of nine DCE questions each. Respondents were randomly assigned to one block of nine choice questions, which were randomly ordered within each block to avoid ordering effects.
Data analyses
Data were managed and analyzed using STATA 16 (Stata Corp., College Station, TX). For descriptive analyses, continuous variables were summarized using the mean, standard deviation, median, and/or range. Categorical data were summarized using frequencies and percentages.
DCE data were analyzed using a random parameters logit model relating choices made to differences in the attribute levels across the alternatives (Citation30). All the levels in each attribute were effects coded (Citation31). The resulting log-odds parameter estimates from the random parameters logit model can be interpreted as preference weights that indicate the relative strength of preferences for each attribute level. A Wald χ2 test was used to determine the statistical significance of differences between adjacent attribute levels for each attribute.
The preference weights were used to calculate the conditional relative importance of each attribute proportional to the others and quantitative estimates of the tradeoff respondents were willing to make for specific changes in attribute levels. The conditional relative attribute importance provides a measure of the overall importance of that attribute relative to the other attributes in the study across the full range of levels for each attribute. The difference between the most- and least-preferred levels of each attribute are summed across attributes, and the sum is scaled to 100. The conditional importance of each attribute is a percentage of this total. A t-test was used to test for differences in the conditional relative attribute importance weights for all possible pairs of attributes.
Minimum acceptable benefit is the level of benefit that offsets the decrease in utility attributable to an increase in risk or a worsening in the levels of another attribute. The models’ estimates for the sample were used to calculate the minimum acceptable benefit as the improvement in itch and the chance of achieving clear or almost clear skin (in percentage points) that were required for respondents to accept a given increase or worsening in the levels of risks and treatment attributes (i.e., longer time until a noticeable itch reduction, greater risk of serious infection, greater risk of developing acne, and possibility of having to use prescription topical steroids).
Similarly, maximum acceptable risk is the mean maximum level of risk of treatment-related AEs that respondents are willing to accept for a given improvement in treatment outcomes for AD. The maximum acceptable risk is calculated as the change in the risk of a treatment-related AE that would exactly offset the perceived benefit of a given improvement in effectiveness. The study calculated maximum acceptable risks for an annual risk of serious infection and a patient’s chance of developing acne in exchange for improvements in other attributes.
Results
Respondents
Of the 213 individuals recruited by physicians who received an invitation email, 204 (95.8%) accessed the survey link; 200 respondents met the inclusion criteria and provided surveys that were considered complete (). At the time of completing the survey, 74.5% of the respondents reported moderate to very severe AD symptoms, 90.5% reported itch within the last seven days, and 39.5% had experienced itch during at least five of the last seven days.
Table 1. Baseline demographics and characteristics.
Overall attribute importance and relative importance given to AD treatment attributes
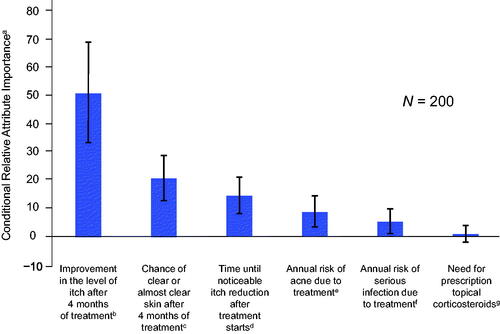
Overall, itch improvement (from a 10% to 100% improvement from baseline) was the most important attribute to respondents according to analyses of conditional relative attribute importance, accounting for almost 50% of the total change in utility possible across the attributes. This was followed by an improvement in the chance of clear or almost clear skin (from 5% to 70%), faster itch relief (from 14 days to 1 day), avoiding 25% risk of acne, avoiding a 2% risk of serious infection, and avoiding the need for prescription topical corticosteroids (). Preferences for all attributes were statistically significant except for the need of prescription topical corticosteroids, suggesting that respondents were not willing to accept worse levels of other attributes for a treatment that did not require the use of topical corticosteroids.
Figure 1. Conditional relative attribute importance for respondents. The conditional relative importance is the difference between the preference weights on the most influential attribute level and the least influential attribute level. These differences are summed across attributes and the sum is scaled to 100. Vertical bars surrounding each relative importance weight estimate denote the 95% confidence interval around the point estimate (computed by the delta method). aA greater value represented a greater importance to patients, conditional on the attributes and levels included in the survey. bImprovement in itch was assessed from levels of 10% to 100%. cChance of clear or almost clear skin was assessed from levels of 5% to 70%. dTime until itch reduction was noticeable was assessed from levels of 1 to 14 days. eAnnual risk of acne due to treatment was assessed from levels of 0% to 25%. fAnnual risk of a serious infection due to treatment was assessed from levels of 0% to 2%. gNeed for topical corticosteroids was assessed from levels of ‘yes’ or ‘no’.
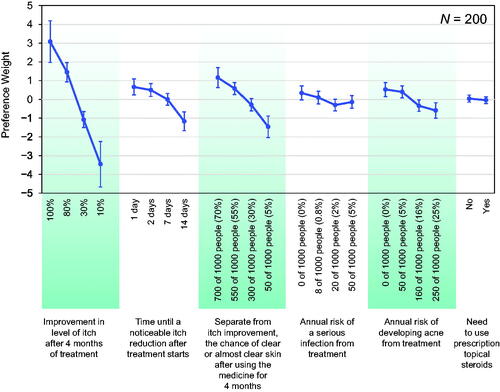
provides the preference weights for each attribute level (see Supplemental Table 1 for numerical weights). In general, among the attribute levels studied, respondents significantly preferred greater itch improvement levels and greater chances of clear or almost clear skin (see Supplemental Table 2 for the numerical differences between changes in preference weights). The change in preference weights for greater itch improvement levels was greater than those for similar changes in the chances of clear or almost clear skin (e.g., a change in the improvement in itch from 10% to 30% was almost as important as an increase in the chance of clear or almost clear skin from 5% to 70%). Respondents were indifferent between 1 day and 2 days until itch relief, though preferences significantly favored one day over seven days (p = .03) and seven days over 14 days (p < .01). The risks of serious infection and developing acne presented in the survey were generally less important than the improvements in itch reduction.
Figure 2. Attribute preference weights for respondents. The parameter estimates are the preference weights corresponding to the effects-coded attribute levels. The preference weights corresponding to the effects-coded variables are categorical variables ranging from −1 to 1. The preference weights corresponding to the effects-coded variables are log odds, which are distributed symmetrically around zero. The vertical bars surrounding each mean preference weight denote the 95% confidence interval of the point estimate (computed by the delta method for the level omitted in estimation for model specification).
Minimum acceptable benefit
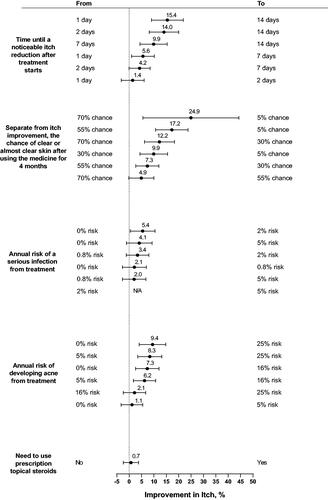
The largest minimum acceptable benefit in percentage-point increase in ‘improvement in itch after 4 months of treatment’ was for a change in chance of clear or almost clear skin (). Respondents on average required an increase in itch improvement of 24.9 percentage points (95% confidence interval [CI] 5.5–44.3) to accept a decreased change of clear or almost clear skin from 70% to 5%. Respondents required on average an increase in itch improvement of 15.4 percentage points (95% CI 9.0–21.8) to accept a delay in ‘time until noticeable itch improvement after treatment starts’ from 1 day to 14 days and an increase in itch improvement of 9.4 percentage points (95% CI 4.0–14.8) to accept an increased risk of acne from 0% to 25%.
Figure 3. Minimum acceptable benefit (MAB) in improvement in level of itch after 4 months of treatment for a given change in treatment attributes (N = 200). MAB is shown as the percentage-point increase in improvement in level of itch after 4 months of treatment with 95% confidence interval. When the 95% confidence interval includes 0, the minimum acceptable benefit is not statistically different from 0. The MAB calculations hold constant the levels of all attributes other than the 1 attribute that is changing. N/A (not applicable) means that the MAB is undefined because none of the attribute levels affected treatment choice.
Maximum acceptable risk
Results from the maximum acceptable risk analyses indicated that respondents on average were willing to accept a >5% serious infection risk to achieve almost every improvement offered in the DCE (). On average, respondents were willing to accept a >5% risk of serious infection for a 20 percentage-point increase in itch reduction from 10% to 30%, a five-day reduction in time to itch improvement from 7 days to 2 days, or a 15 percentage-point increase in the chance of achieving clear or almost clear skin from 55% to 70%. On average, respondents were also willing to accept a >25 percentage-point increased risk of acne for a 20 percentage-point increase in itch reduction from 10% to 30%, a 5.5 percentage-point increased risk for acne with a one-day reduction in time to itch improvement from 2 days to 1 day, or an 11.7 percentage-point increased risk of acne for a 15 percentage-point increase in the chance of achieving clear or almost clear skin from 55% to 70%.
Table 2. Maximum acceptable risk of serious infection from treatment for a given change in treatment attributes (N = 200).
Discussion
AD causes intense pruritus and skin pain that lead to substantial negative impacts on patients’ HRQoL (Citation2,Citation3,Citation5). Given that there is currently no cure for AD, patients must contemplate tradeoffs between efficacy, side effects, mode of administration, and other attributes of competing treatments. Here, we conducted a DCE to quantify patients’ preferences among a set of benefits and risks associated with AD therapies. Conditional on the set of attributes and levels included in this study, the most important attributes for respondents were itch reduction and greater probability of skin clearance, followed by time to onset of itch relief, risk of acne, and risk of serious infection; avoiding the need for prescription topical corticosteroids was ranked as the least important attribute.
Results from other recent DCE studies evaluating AD treatments consistently identified itch reduction as one of the most important treatment attributes among those assessed within each study (Citation25–27). In a DCE study of 323 patients in Japan, findings indicated the most important treatment benefits reported were risk of mild adverse effects, time until response, and efficacy of itch reduction (Citation25). In another DCE survey of 404 respondents in France, Spain, and the UK, findings demonstrated that the most valued attribute was itch improvement (from 20% to 50%), followed by decreased risk of serious infections (from 6% to 0%), decreased risk of eye inflammation (from 20% to 0%), and improvement in the chance of achieving clear or almost clear skin (from 10% to 40%) (Citation27). In a DCE study of 320 adult patients with AD in the US and UK, results indicated that itch relief onset and skin clearance probability were the most valued respondent benefits (Citation26). Our findings were generally consistent with those reported in the other DCE studies; in addition, our study evaluated two different dimensions of itch (degree of itch reduction and itch relief onset).
Respondents prioritized a reduction in itch over other attributes in our study, including the chance of clear or almost clear skin, and they were willing to take on AE risks to achieve itch reduction. The findings highlight the intense itch experienced by patients with moderate-to-severe AD and may explain the selection of additional discomfort or risk in exchange for greater itch reduction. Altogether, armed with the findings from this study, clinicians can effectively counsel their own patients on the most effective and preferred therapy options, based on the preferences of nearly 200 patients with the same diagnosis. Patients’ preferences are integral to inform shared decision-making between patients and their physicians and to develop appropriate AD treatment plans. Shared decision-making is especially important for treating dermatologic conditions, including AD, because of the high psychosocial burden and the wide range of treatment options currently available (Citation32,Citation33).
While our study featured several unique facets, the limitations of this study, including some inherent to DCE surveys, must be considered. We employed a well-vetted approach to quantifying patient’s perspectives on decision points, with a rigorous qualitative evaluation of our survey. Nonetheless, respondent characteristics may differ from the broader AD population. The data collected in DCEs are based on responses to hypothetical choice profiles. Attempts were made to limit potential hypothetical bias by constructing choice questions that mimic realistic clinical choices as closely as possible and map clearly onto clinical evidence. However, DCE surveys can accommodate only a limited number of treatment attributes; our study includes only a subset of the outcomes and burdens associated with AD treatment. It is also plausible that some respondents may have been familiar with the treatment AEs selected for the study, which may have caused some to respond differently than would respondents who were unfamiliar with the treatment AEs. Our findings are dependent on the attributes and the range of attribute levels selected for this study, and results may differ if other ranges or levels are selected. Selection bias is a final potential confound. The general population likely possesses varied internet access and, thus, results may differ from respondents. We are neither able to measure nor control for this possible bias.
Respondents with moderate-to-severe AD prefer systemic treatments that provide greater itch improvement, a greater likelihood of clear or almost clear skin, and faster itch reduction for the range of improvements studied and were willing to trade increased serious infection or acne risk for greater or more rapid itch reduction and skin clearance. Given the selection of AD medications currently available, the results suggest that careful discussion about patient preferences could help improve patient satisfaction with treatment.
Supplemental Material
Download PDF (276.9 KB)Acknowledgements
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. AbbVie and authors thank all respondents who participated in this study. Medical writing support, funded by AbbVie, was provided by Callie A. S. Corsa, PhD, and Lamara D. Shrode, PhD, ISMPP CMPP™, of JB Ashtin, who developed the first draft based on an author-approved outline and assisted in implementing author revisions. JB Ashtin adheres to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations.
Disclosure statement
Dr. Kwatra is an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals. He has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Lio has received research grants/funding from AbbVie, AOBiome, the National Eczema Association, and Regeneron/Sanofi Genzyme. He participates in speaker’s bureaus for Eli Lilly, Galderma, Incyte, L’Oreal, LEO Pharma, Pfizer, and Regeneron/Sanofi Genzyme. He reports payments for serving as a consultant or participating on advisory boards for AbbVie, Almirall, Amyris, AOBiome, Arbonne, Aslan, Bodewell, Bristol Myers Squibb, Burt’s Bees, Concerto Biosciences (stock options), Dermavant, Eli Lilly, Exeltis, Galderma, IntraDerm, Johnson & Johnson, Kimberly-Clark, Kiniksa, LEO Pharma, L’Oreal, Menlo Therapeutics, Merck, Micreos (stock options), My-Or Diagnostics, Pierre-Fabre, Pfizer, Realm Therapeutics, Regeneron/Sanofi Genzyme, Sibel Health, Theraplex, UCB, Unilever, and Verrica. In addition, Dr. Lio has a patent pending for a Theraplex product and receives royalty payments for the patent. He is also a board member and scientific advisory committee member of the National Eczema Association. Dr. Weidinger is a speaker, advisory board member, and/or investigator for AbbVie, Almirall, Eli Lilly, Galderma, Kymab, LEO Pharma, Pfizer, Regeneron, and Sanofi. He has received research grants from La Roche Posay, LEO Pharma, Pfizer, and Sanofi Germany. Drs. Calimlim, Ladizinski, and Vigna are full-time employees of AbbVie Inc. and may own AbbVie stock and/or stock options. Dr. Botha is a former employee of RTI Health Solutions, which received funding from AbbVie Inc. to conduct the study. He was an employee of RTI Health Solutions at the time the study was conducted. Dr. Mansfield is a current employee of RTI Health Solutions, which received funding from AbbVie Inc. to conduct the study.
Data availability statement
AbbVie is committed to responsible data sharing regarding the studies we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.html.
Additional information
Funding
References
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):1–8.
- Whiteley J, Emir B, Seitzman R, et al. The burden of atopic dermatitis in US adults: results from the 2013 National Health and Wellness Survey. Curr Med Res Opin. 2016;32(10):1645–1651.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498.
- Dalgard FJ, Gieler U, Tomas-Aragones L, et al. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–991.
- Beikert FC, Langenbruch AK, Radtke MA, et al. Willingness to pay and quality of life in patients with atopic dermatitis. Arch Dermatol Res. 2014;306(3):279–286.
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140(3):633–643.
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878.
- Silverberg JI, Thyssen JP, Fahrbach K, et al. Comparative efficacy and safety of systemic therapies used in moderate-to-severe atopic dermatitis: a systematic literature review and network meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(9):1797–1810.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343.
- Simpson EL, Silverberg JI, Nosbaum A, et al. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol. 2021;22(5):693–707.
- Waldman RA, DeWane ME, Sloan B, et al. Characterizing dupilumab facial redness: a multi-institution retrospective medical record review. J Am Acad Dermatol. 2020;82(1):230–232.
- Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther. 2023;13(2):651–660.
- Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a Real-Life French Multicenter Adult Cohort. J Am Acad Dermatol. 2019;81(1):143–151.
- Hagino T, Saeki H, Fujimoto E, et al. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023.
- Rogner D, Biedermann T, Lauffer F. Treatment of atopic dermatitis with baricitinib: first real-life experience. Acta Derm Venereol. 2022;102:adv00677.
- Uchiyama A, Fujiwara C, Inoue Y, et al. Real-world effectiveness and safety of baricitinib in Japanese patients with atopic dermatitis: a single-center retrospective study. J Dermatol. 2022;49(4):469–471.
- Clinicaltrials.gov. A study to learn about the study medicine (called abrocitinib) in adult patients with moderate to severe atopic dermatitis: NCT05250115; 2023 [cited 2023 Apr 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT05250115
- Clinicaltrials.gov. A study to learn about the study medicine (called Cibinqo) in people with atopic dermatitis: NCT05391061; 2023 [cited 2023 Apr 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT05391061
- Clinicaltrials.gov. BioDay registry: data collection regarding the use of new systemic treatment options in patients with atopic dermatitis: NCT03549416; 2023 [cited 2023 Apr 3]. Available from: https://clinicaltrials.gov/ct2/show/NCT03549416
- Soekhai V, de Bekker-Grob EW, Ellis AR, et al. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226.
- Carrascosa Carrillo JM, Baselga Torres E, Gilaberte Calzada Y, et al. Quantifying physician preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. Dermatol Ther. 2022;12(5):1197–1210.
- Okubo Y, Ho KA, Fifer S, et al. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J Dermatolog Treat. 2020;31(8):821–830.
- Boeri M, Sutphin J, Hauber B, et al. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J Dermatolog Treat. 2022;33(3):1449–1458.
- Thomas C, Raibouaa A, Wollenberg A, et al. Patient preferences for atopic dermatitis medications in the UK, France and Spain: a discrete choice experiment. BMJ Open. 2022;12(8):e058799.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR good research practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315.
- Hensher DA, Rose JM, Greene WH. Applied choice analysis. Cambridge (UK): Cambridge University Press; 2005.
- Tan J, Linos E, Sendelweck MA, et al. Shared decision making and patient decision aids in dermatology. Br J Dermatol. 2016;175(5):1045–1048.
- van der Kraaij GE, Vermeulen FM, Smeets PMG, et al. The current extent of and need for shared decision making in atopic dermatitis and psoriasis in The Netherlands: an online survey study amongst patients and physicians. J Eur Acad Dermatol Venereol. 2020;34(11):2574–2583.

