Abstract
Introduction
This post hoc analysis assessed association between scalp hair regrowth and improvements in health-related quality of life (HRQoL) and psychological burden in patients with severe alopecia areata (AA).
Methods
Data were pooled from two phase-3 trials (N = 1200). Patients randomized to once-daily placebo, baricitinib 2-mg, or 4-mg were analyzed independently of treatment allocation, and categorized according to scalp hair regrowth (at Week 36): meaningful regrowth (Severity of Alopecia Tool (SALT) score ≤20); intermediate regrowth (≥30% SALT improvement [SALT30] at any post-baseline visit to Week 36, but SALT score > 20 at Week 36); no/minimal regrowth (never achieved SALT30). Skindex-16 for AA score change-from-baseline and proportion of patients with baseline Hospital Anxiety and Depression Scale (HADS) scores ≥8 that shifted to <8 (normal) were assessed.
Results
Patients with meaningful regrowth achieved greater improvements in all Skindex-16 AA domains versus no/minimal regrowth. More patients with meaningful versus no/minimal regrowth shifted from HADS ≥8 to <8 (anxiety:46.8% versus 26.4%; depression:52.3% versus 24.0%). Improvements occurred with intermediate regrowth but to a lesser extent versus meaningful regrowth.
Conclusions
Patients with severe AA and scalp hair regrowth at Week 36 experienced greater improvements in HRQoL and anxiety and depression versus patients with no/minimal regrowth. The highest benefit was observed in patients with meaningful regrowth (SALT score ≤20).ClinicalTrials.gov listing: NCT03570749 and NCT03899259
Introduction
Alopecia areata (AA), an autoimmune disorder resulting in hair loss on the scalp and/or body, is frequently associated with impaired health-related quality of life (HRQoL) and psychological burden (Citation1,Citation2). There are limited data on the impact of hair regrowth on HRQoL and psychological burden (Citation3). Baricitinib, an oral Janus kinase (JAK)1/JAK2 inhibitor, is the first Food and Drug Administration–approved medication for adults with severe AA and is also approved in Europe and Japan. In two phase 3 clinical trials, baricitinib demonstrated superiority over placebo in hair regrowth after 36 weeks of treatment (Citation4). Significant improvements in HRQoL and psychological symptoms were also observed with baricitinib (Citation5). This post hoc analysis assessed the association between scalp hair regrowth and improvement in HRQoL and psychological burden in patients with severe AA.
Methods
Study design
BRAVE-AA1 (NCT03570749) and BRAVE-AA2 (NCT03899259) are double-blind, parallel-group, randomized, placebo-controlled trials involving 169 centers in 10 countries. Adults with severe AA (defined as hair loss of ≥50% of the scalp) were initially randomized to placebo, baricitinib 2-mg, or baricitinib 4-mg for 36 weeks. Both trials have identical eligibility criteria and identical primary and key secondary objectives for the 36-week placebo-controlled period. Detailed study design information has been published previously (Citation4). The trials were conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the research protocols were approved by each center’s institutional review board or ethics committee. All patients provided written informed consent.
Outcomes
Scalp hair loss was measured with the Severity of Alopecia Tool (SALT). The SALT score is a weighted sum of the percent of hair loss in the four quadrants of the scalp. Absolute SALT scores range from 0 (no scalp hair loss) to 100 (complete scalp hair loss) (Citation6). A SALT score ≤20 (i.e. scalp hair loss of 20% or less) was identified by physicians and patients with severe AA as meaningful scalp hair regrowth (Citation7). SALT30 means at least a 30% improvement from the baseline in the SALT score. SALT30 had been previously used as a minimum threshold for efficacy assessment (Citation8).
The Skindex-16 was used to assess the effects of AA on HRQoL (Citation9). With the authorization of the original authors and license holders, the Skindex-16 was adapted to assess HRQoL in adults with AA by changing ‘your skin condition’ to ‘your alopecia’ or ‘your scalp’ (Skindex-16 AA) (Citation10). Skindex-16 AA consists of 3 domains (symptoms, emotions, and functioning) with normalized scores ranging from 0 (no effect) to 100 (effect experienced all the time).
The Hospital Anxiety and Depression Scale (HADS) was selected to measure psychological distress in adult patients with severe AA. The HADS determines on two separate scales the levels of anxiety (HADS-A) and depression (HADS-D) that a patient has experienced over the past week. Scores for each scale range from 0 to 21, with higher scores indicating greater levels of anxiety or depression (Citation11,Citation12). The HADS was used to identify patients with borderline or abnormal (B/AB) scores for anxiety or depression at baseline (HADS-A or HADS-D ≥ 8) with scores of <8 considered normal (Citation11,Citation12).
Statistical analyses
For this work, we pooled phase 3 data from the placebo-controlled periods of the two trials and conducted analyses independently of treatment allocation. Patients were categorized into three mutually exclusive categories according to scalp hair regrowth at Week 36: (1) meaningful regrowth: a SALT score ≤20 by Week 36; (2) intermediate regrowth: at least 30% improvement from baseline in SALT score (SALT30) at any post-baseline visit up to Week 36 but with a SALT score >20 at Week 36; and (3) no/minimal regrowth: patients who had never achieved SALT30 up to Week 36.
At Week 36, the change from baseline in each Skindex-16 AA domain score was analyzed using analysis of covariance with modified last observation carried forward imputation for missing data (data after permanent study drug discontinuation were censored and not carried forward). The percentages of patients for whom scores shifted from B/AB to normal HADS-A and HADS-D scores were analyzed at Week 36 using logistic regression with non-responder imputation.
Results
There were 1200 patients in the pooled dataset with 256 (21.3%) in the meaningful regrowth group, 268 (22.3%) in the intermediate regrowth group, and 676 (56.3%) in the no/minimal regrowth group. Baseline demographic and clinical characteristics are reported in . The vast majority of patients (94.5%) in the meaningful regrowth group were receiving baricitinib treatment. Most patients on placebo (81.1%) were in the no/minimal regrowth group. The mean duration of disease (time since AA onset) and mean duration of the current episode of AA-related hair loss were shorter in the meaningful regrowth group than in the two other groups. The severity of scalp hair loss (mean SALT score) at baseline was also lower in the meaningful regrowth group.
Table 1. Baseline demographics and clinical characteristics.
Skindex-16 AA individual domain scores and the percentages of patients with B/AB HADS scores were generally similar across groups at baseline. Skindex-16 AA emotions score was the highest of the three domain scores at baseline. Approximately one-third of patients (33% to 37% across regrowth groups) had B/AB anxiety scores and 15% to 19% of patients had B/AB depression scores at baseline.
At Week 36, patients in the meaningful regrowth group achieved significantly greater improvements in all Skindex-16 AA domains versus those in the no/minimal regrowth group (symptoms p < 0.05; emotions and functioning p < 0.001). In all groups, the greatest improvements were seen in the domain of emotions ().
Figure 1. Least square mean change from baseline to Week 36 in Skindex-16 AA domain scores (A) and percentage of patients in whom scores shifted from B/AB to normal HADS-a and HADS-D scores at Week 36 (B). *p < 0.05; **p < 0.01; ***p < 0.001 vs no or minimal regrowth. B/AB: borderline/abnormal; HADS-A: Hospital Anxiety and Depression Scale-anxiety; HADS-D: Hospital Anxiety and Depression Scale-depression; SALT: Severity of Alopecia Tool.
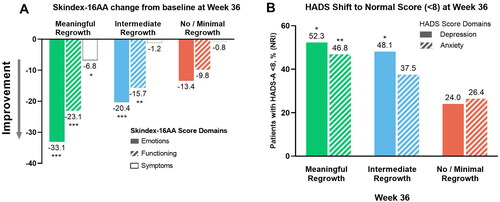
Among patients with B/AB anxiety or depression scores at baseline, significantly more patients in the meaningful regrowth group versus those in the no/minimal regrowth group had HADS scores that shifted to normal at Week 36 (depression p < 0.05; anxiety p < 0.01; ). At Week 36, improvements in Skindex-16 AA and HADS scores were also observed in the intermediate and no/minimal regrowth groups but to a lesser extent.
Discussion
Our analysis shows that patients with severe AA and meaningful scalp hair regrowth (SALT score ≤20) experienced greater improvements in HRQoL and symptoms of anxiety and depression than patients with no/minimal scalp hair regrowth. Patients with intermediate scalp hair regrowth at Week 36 also experienced improvements compared with those in the no/minimal regrowth group but to a lesser extent than those with meaningful hair regrowth. These results seem aligned with recent findings from a study of two other JAK inhibitors, in which medium-to-large correlations were seen between scores on the SALT and Alopecia Areata Symptom Impact Scale, an instrument designed to assess the quality of life and symptoms and their impact on patients with AA (Citation13).
In our analysis, patients with meaningful scalp hair regrowth at Week 36 had a shorter mean duration of disease and current episode of hair loss and a lower mean SALT score at baseline. On the other hand, Skindex-16 AA and HADS scores were similar among the three groups at baseline. Conflicting results have been published regarding the relationship between the severity of scalp hair loss and HRQoL impairment, which underscores that there are complexities yet to be understood about the impacts of the disease on HRQoL (Citation1,Citation14).
Among the Skindex-16 AA domains, scores were the lowest for the symptoms domain at baseline across groups, which is consistent with the fact that patients who enrolled in the BRAVE-AA trials had relatively stable disease with no spontaneous improvement (≤10-point reduction in the SALT score) during the 6 months preceding enrollment (Citation4). Symptoms of skin discomfort such as itching or burning are rarely reported by patients with AA, except in situations of active hair loss or hair regrowth (Citation15). Conversely, greater baseline impairment was observed in the emotions and functioning domains of Skindex-16 AA, offering greater room for improvement. Studies have clearly established that AA can have a profound psychological and psychosocial impact on patients, which translates into significant impairment in HRQoL (Citation1). Only a limited number of patients presented with B/AB HADS scores at baseline. This might be related to the exclusion of patients with significant uncontrolled neuropsychiatric disorders (Citation4).
Our analysis presents some limitations. First, longer treatment duration may be needed to assess the full impact of hair regrowth on HRQoL and symptoms of anxiety and depression. Second, we have reported the association of improvements in Skindex-16 AA and HADS with scalp hair regrowth only. The contribution of hair regrowth on different body sites, especially eyebrows and eyelashes, to the improvements in HRQoL and psychological burden needs to be further assessed. Finally, comparisons performed in this post hoc analysis are of an exploratory nature.
In summary, this post hoc analysis showed that clinically meaningful scalp hair regrowth (SALT score ≤20) achieved higher levels of improvement in HRQoL and measures of anxiety and depression versus patients with no/minimal growth. Psychosocial distress is a key feature of AA and these data suggest that hair regrowth is a reasonable explanation for the improvements in HRQoL and psychological well-being observed after successful treatment in patients with severe AA.
Ethical statement
IRB approval status: BRAVE-AA1 was first approved by Advarra IRB on August 13, 2018. BRAVE-AA2 was first approved by Quorum Review IRB on February 19, 2019. Study participants provided written informed consent prior to the start of study procedures.
Acknowledgments
The authors would like to thank all patients, investigators, and study staff who participated in these trials. Medical writing and editorial support were provided by Kathy Oneacre, MA, of Syneos Health, funded by Eli Lilly and Company.
Disclosure statement
Bianca Maria Piraccini reports lecture fees from Pierre Fabre-Ducray, Difa Cooper, and Dercos-L’Oreal and advisory fees from Pfizer, Eli Lilly Italy, ISDIN, Legacy Healthcare, and Almirall.
Manabu Ohyama receives lecture fees from Eli Lilly Japan K.K., advisory fees from Eli Lilly Japan K.K., Pfizer Japan Inc., Janssen Pharmaceutical K.K., Taisho Pharmaceutical Co., and RHOTO Pharmaceutical Co., and research grants not related to the current work from Maruho Corp., Sun Pharma Japan Ltd, ADVANTEST Co., and Shiseido Co.
Brittany Craiglow reports honoraria and/or fees from Concert Pharmaceuticals, Eli Lilly, Incyte, Pfizer, Regeneron, and Sanofi-Genzyme.
Anthony Bewley has had ad hoc consultancy agreements with AbbVie, Celgene Corporation, Galderma, Janssen Pharmaceuticals, LEO Pharma, Novartis and Stiefel (a GSK company).
Yuxin Ding, Yun-Fei Chen, Yves Dutronc, Evangeline Pierce, and Frederick Durand are employees and stockholders at Eli Lilly and Company.
Arash Mostaghimi reports consulting fees from Eli Lilly and Company, Pfizer, Equillium, hims and hers, ACOM, Digital Diagnostics, AbbVie, and Concert.
Data availability statement
Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and European Union, and after primary publication acceptance, whichever is later. No expiration date for data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Additional information
Funding
References
- Liu LY, King AB, Craiglow GB. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75(4):1–4.e3. doi: 10.1016/j.jaad.2016.04.035.
- Toussi A, Barton VR, Le ST, et al. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85(1):162–175. doi: 10.1016/j.jaad.2020.06.047.
- Messenger A, Harries M. Baricitinib in alopecia areata. N Engl J Med. 2022;386(18):1751–1752. doi: 10.1056/NEJMe2203440.
- King B, Ohyama M, Kwon O. et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–1699. doi: 10.1056/NEJMoa2110343.
- Piraccini BM, Ohyama M, Craiglow B, et al. The contribution of hair regrowth to health-related quality of life improvement in patients with alopecia areata treated with baricitinib or placebo. Paper presented at: World Congress for Hair Research 2022; 2022 November 18–21; Melbourne, Australia.
- Olsen EA, Hordinsky MK, Price VH. et al. Alopecia areata investigational assessment guidelines–part II. National alopecia areata foundation. J Am Acad Dermatol. 2004;51(3):440–447. doi: 10.1016/j.jaad.2003.09.032.
- Wyrwich KW, Kitchen H, Knight S, et al. The alopecia areata investigator global assessment scale: a measure for evaluating clinically meaningful success in clinical trials. Br J Dermatol. 2020;183(4):702–709. doi: 10.1111/bjd.18883.
- King B, Ko J, Forman S, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol. 2021;85(4):847–853. doi: 10.1016/j.jaad.2021.05.050.
- Chren MM. The skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30(2):231–236, xiii. doi: 10.1016/j.det.2011.11.003.
- Chren MM. SKINDEX-16 for alopecia areata (SKINDEX-16 for AA). 2018. Available from: https://eprovide.mapi-trust.org/instruments/skindex-16-for-alopecia-areata#contact_and_conditions_of_use.
- Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x.
- Winnette R, Banerjee A, Sikirica V, et al. Characterizing the relationships between patient-reported outcomes and clinician assessments of alopecia areata in a phase 2a randomized trial of ritlecitinib and brepocitinib. J Eur Acad Dermatol Venereol. 2022;36(4):602–609. doi: 10.1111/jdv.17909.
- Aldhouse NVJ, Kitchen H, Knight S, et al. You lose your hair, what’s the big deal?’ I was so embarrassed, I was so self-conscious, I was so depressed”: a qualitative interview study to understand the psychosocial burden of alopecia areata. J Patient Rep Outcomes. 2020;4(1):76. doi: 10.1186/s41687-020-00240-7.
- Edson-Heredia E, Cutts K, Bethesda E, et al. The relationship between the degree of scalp hair loss and health-related quality of life among patients with alopecia areata. J Am Acad Dermatol. 2022;87(3):AB120. Suppl AB120, doi: 10.1016/j.jaad.2022.06.512.

