Abstract
Introduction
Atopic dermatitis (AD) exhibits difference in immune polarization between Caucasians and Asian races due to which an evaluation of the efficacy and safety of Pimecrolimus (PIM) in Asian population is called for. The current study addresses the need via a sub-group analysis of the PETITE study (NCT00120523) to evaluate the safety and efficacy of PIM in Chinese infants.
Materials and methods
Patients with AD (≥3 months–<12 months of age) were randomized in a 1:1 ratio to either PIM 1% cream or topical corticosteroids (TCS). The primary endpoint was safety. The secondary endpoint was efficacy.
Results
120 patients were randomized to either PIM 1% or TCS (n = 61 for PIM, n = 59 for TCS). The most often reported adverse events were reported by similar proportions of patients treated with PIM or TCS. There was a progressive increase in overall IGA treatment success in infants treated with PIM (82.9%, p < .05, 95% CI: 70.4, 95.3) after 26 weeks which was comparable to the TCS group (88.5%, p < .05, 95% CI: 79.8, 97.1).
Conclusion
PIM showed an early and sustained efficacy in the Chinese sub-population with a substantial corticosteroid-sparing effect in patients with AD.
1. Introduction
Atopic dermatitis (AD) is a chronic, pruritic inflammatory disease affecting 10–20% of the pediatric population (Citation1). The disease is characterized by pruritus, skin dryness, and other skin lesions such as serous exudate, excoriation, papules, and lichenification. Although the exact cause of AD is unknown, several studies have indicated that the skin epithelial function and innate/adaptive immune responses might be the two main biological pathways responsible for AD etiology (Citation2). The average onset age for AD in China is 0.86 ± 3.87 years with a prevalence of 12.94% among the pediatric population (Citation3). The incidence of AD is rising in China especially in children (Citation4).
Current treatment standards for AD in infants include the use of emollients and topical corticosteroids (TCS). However, the long term use of TCS is not recommended in infants due to the associated risks and side effects. Long term use of TCS may cause local side effects like skin infections, impairment of the epidermal barrier function, and skin atrophy. Also, ‘corticosteroid phobia’ is increasingly recognized as a significant factor contributing to poor TCS treatment adherence (Citation5,Citation6).
Topical calcineurin inhibitors (TCI) have been recommended by the European expert panel for the long term treatment of AD in infants (Citation7). Pimecrolimus 1% cream (PIM) is a TCI that has been found to be a safe and better alternative to TCS in infants (Citation7). Although PIM was approved in early 2000s, a black-box warning was mandated due to the potential risk of malignancies in 2005 (Citation8). However, substantial evidence was shown in favor of the safety and efficacy of PIM in infants (Citation8). A recent meta-analysis has confirmed with moderate certainty that the use of TCIs like PIM or tacrolimus is not associated with risk of cancer (Citation9). Use of PIM cream in infants aged three months and above with AD is already approved in several countries like Australia, Brazil, Canada, India, Indonesia, Israel, New Zealand, Philippines, Russia, and Thailand. Recently, PIM has been approved in infants (≥3 months) for the treatment of AD in Europe. Pimecrolimus has also been recommended for the treatment of AD in infants in southeast Asia by experts (Citation5). The guidelines in China recommend PIM in children with mild to moderate AD. Other therapies include administration of oral antihistamines, oral gluco-corticosteroids, immunosuppressants and JAK-inhibitors (Citation10). A five-year randomized trial by Sigurgeirsson et al. (PETITE study) involving 2418 infants has shown the safety and efficacy of PIM over TCS in the treatment of AD (Citation11). Other studies have also showed the safety and efficacy of PIM in infants for the treatment of AD (Citation12–17). Recently, experts from China have proposed a TCS-sparing treatment algorithm for the management of infants, children, adolescents, and adults with mild-to-moderate AD in China (Citation18).
AD is believed to exhibit difference in immune polarization between Caucasians and Asian races (higher activation of Th17/Th22 cells in Asian patients) (Citation5,Citation19,Citation20). Caucasians, on the other hand, did not show Th-17 axis activation (Citation21). Asian patients with AD have higher interleukin 19 (IL-19) which increases IL-17’s effect on keratinocytes, that play an important role in atopic skin inflammation (Citation22). Studies have shown that AD harbors different endotypes across different age groups and ethnicities and according to IgE levels and filaggrin mutation status that include European American versus Asian patients (Citation21). Due to these differences in the population an evaluation of the efficacy and safety of PIM in Asian population is warranted, and the current study addresses this need. This study is a sub-group analysis of the PETITE study. The Chinese population who was part of the global PETITE study were included in this sub-group analysis and evaluated for the safety and efficacy of PIM in Chinese infants. The study compared the results of the Chinese sub-group analysis with the global PETITE study data.
2. Methods
The following is a sub-group analysis of the five-year multicenter, open-label, randomized, parallel group study done by Sigurgeirsson et al. (Citation11). The global study enrolled 2418 infants to compare the safety and efficacy of PIM over TCS for the treatment of AD. Patients enrolled at the study centers in China were included in this subgroup analysis. Patients were enrolled into the study between April 2004 and October 2005. The primary endpoint was safety. The secondary endpoint was efficacy which was evaluated by investigators during clinic visits using the Investigator Global Assessment (IGA: range 0–5) for the whole body with a score of 0 (clear) or 1(almost clear) indicating treatment success, and the Total Body Surface Area affected (TBSA) by inflammation (percent TBSA covered with AD skin lesions). The duration of the study was five years. For the subgroup analysis safety population was equal to the intent-to treat population (ITT).
2.1. Study design
This is a sub-group analysis of the PETITE study (www.clinicaltrials.gov identifier NCT00120523) published by Sigurgeirsson et al. (Citation11). Patients were randomized in a 1:1 ratio to either PIM 1% cream or TCS (low potency, e.g., hydrocortisone 1%; or medium potency, e.g., hydrocortisone butyrate 0.1% cream/ointment used according to the country’s label with potency selected by the investigator). Randomization was stratified by center and age group (3–6 and 6–, 12 months) using a validated Interactive Voice Response System. Study medication was started immediately after randomization and continued until complete AD clearance or for as long as allowed by the label of the specific TCS. Medication was reinitiated at the occurrence of first signs and symptoms of AD flares. In both the treatment groups, investigators explained to caregivers of patients what constituted disease worsening and, to patients in the PIM group, when to stop PIM and start using a TCS, as a rescue medication for an exacerbation not controlled by PIM. The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the Independent Ethics Committee or Institutional Review Board for each center. Caregivers provided written informed consent for infants’ participation in the study.
2.2. Study population
Patients were enrolled into the study between April 2004 and October 2005. Eligible infants were aged ≥3 to 12 months. AD was diagnosed according to the criteria of Seymour et al. (Citation23) (because these criteria were developed for patients aged ≤ 2 years) with disease affecting ≥ 5% of the TBSA. Patients had an IGA score of 2 or 3 (scale range: 0–5), indicating mild-to-moderate disease. Key exclusion criteria were: (i) use of systemic corticosteroids, (ii) use of immunosuppressants, cytostatic drugs, (iii) phototherapy within four weeks of the first application of study medication, (iv) topical tacrolimus ointment or PIM within two weeks, and (v) topical therapy for AD such as TCSs within three days. Immunocompromised patients and those with a history of malignant disease, active acute viral skin infection, or clinically infected AD were also excluded.
2.3. Primary endpoint
2.3.1. Safety analysis
All adverse events (AE) were coded by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (version 13.1). Growth rate was assessed by measuring height and weight at each visit. AEs recorded for the PIM group may have occurred either during treatment with PIM or with TCS for a flare.
2.4. Secondary endpoint
2.4.1. Efficacy analysis
Efficacy was evaluated by investigators during clinic visits using the IGA (range 0–5) for the whole body with a score of 0 (clear) or 1(almost clear) indicating treatment success, and the TBSA by inflammation. Special consideration was given to facial AD since face is more sensitive than other part of the body (Citation24).
2.5. Statistical analysis
2.5.1. Sample size determination
There was no formal sample size determination for this analysis since this was a sub-group analysis of the PETITE study (Citation11). All infants who were enrolled in the PETITE study from the study sites in Greater China were included in the sub-group analysis.
2.5.2. Statistical methods
Adverse event data was summarized descriptively by system organ class and preferred term. Efficacy data i.e., overall, IGA, facial IGA and TBSA observed values were summarized descriptively.
3. Results
3.1. Study population
3.1.1. Disposition
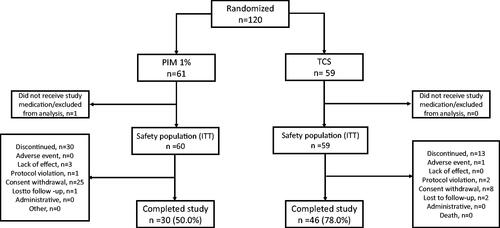
The PETITE study, which was a pivotal study of Pimecrolimus in pediatric patients with AD, included a total of 2418 patients. Of these patients, 119 patients were part of the Chinese subgroup analysis. In the Chinese subgroup analysis, patients who were randomized to either PIM 1% or TCS were evaluated (PIM, n = 60; TCS, n = 59) ().
3.1.2. Demographics and baseline characteristics
The baseline characteristics () were comparable between the treatment groups, except for higher number of PIM patients had an IGA of 3 (moderate disease) compared to the patients treated with TCS (65.0% vs 49.2%).
Table 1. Baseline demographics of the Chinese sub-group population (TCS: Topical corticosteroid; PIM: Pimecrolimus 1%).
3.2. TCS and PIM exposure
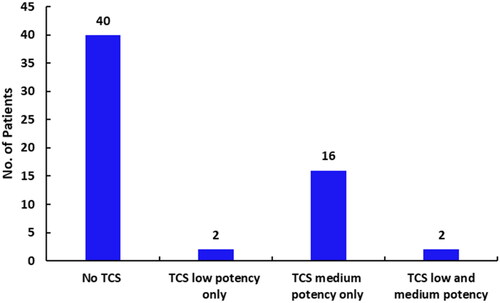
The extent of exposure [mean (standard deviation, s.d.)] to PIM was 207.9(281.69) days and to TCS was 354.4(368.9) days. More than 65% of the patients who used only PIM did not use TCS as rescue medication. In the subjects requiring rescue medication, exposure to both PIM and TCS was 28(82) days ().
3.3. Safety
Treatment-emergent AEs were reported by 106 (89.1%) patients in the overall safety population. 52 (86.7%) patients treated with PIM and 54 (91.5%) patients treated with TCS reported treatment-emergent AEs. The most frequently reported AEs (≥30% in any treatment group) () were nasopharyngitis, pyrexia, diarrhea, upper respiratory tract infection and cough, which are typical childhood disorders. These AEs were reported by similar proportions of patients treated with PIM or TCS. SAEs were reported by 6 (10.0%) patients treated with PIM and 11 patients (18.6%) treated with TCS. There was no death reported. No malignant or benign neoplasm was reported during the study for the Chinese population.
Table 2. Number of patients (%) with adverse events (≥ 30%) by preferred term and treatment group during the treatment period—Chinese sub-group population.
3.4. Efficacy
3.4.1. Overall, IGA treatment success (total IGA 0 or 1)
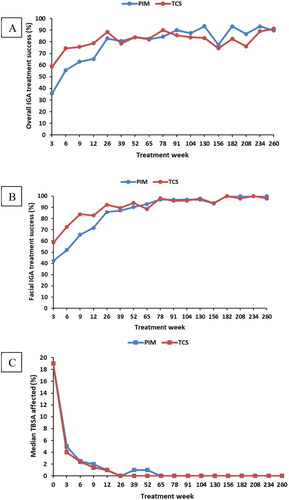
None of the patients in the Chinese study population had an overall IGA of 0 or 1 at baseline. There was a progressive increase in overall IGA treatment success in infants treated with PIM (82.9%, p < .05, CI: 70.4, 95.3) after 26 weeks which was comparable to the TCS group (88.5%, p < .05, CI: 79.8, 97.1) . The overall IGA treatment success at year 5 for PIM was 89.7% (p < .05, CI: 78.6, 100.0) which was comparable to TCS 91.3% (p < .05, CI: 83.2, 99.4) ().
3.4.2. Facial IGA treatment success (facial IGA 0 or 1)
There was a progressive increase in facial IGA treatment success, in infants treated PIM (85.7%, p < .05, CI: 74.1, 97.3) after 26 weeks which was similar to the TCS group (92.3%, p < .05, CI: 85.1, 99.6). The overall facial IGA treatment success at year 5 for PIM was 100% while for TCS it was 97.8% ().
3.4.3. TBSA affected
The TBSA affected by inflammation decreased with treatment and PIM had similar efficacy like TCS for the entire duration (3 weeks to 260 weeks). By week 3, the median TBSA affected by AD decreased from 19% at baseline to 5% in PIM and to 4% in TCS. In the PIM group 0% TBSA was achieved after 1.5 years similar to the overall study population whereas in the TCS this was already achieved after 0.5 years ().
4. Discussion
Atopic dermatitis (AD) primarily affects infants and young children. Unique mutations in the filaggrin (FLG) gene have been identified in Asian populations associated with AD, most notably c.3321delA, whereas R501X and 2282del4 mutations are commonly found in European population (Citation25). This may lead to the production of higher IL-17 cells in Asian population. Although TCSs are often prescribed, non-corticosteroid treatments are needed because compliance with TCSs is poor due to concerns about their side effects. The PETITE study, which was a pivotal study of Pimecrolimus in pediatric patients with atopic dermatitis (Citation11), used a unique real-world design in which TCSs were used according to their label. The caregivers of infants randomized to treatment with PIM had ready access to short-term TCSs as rescue medication if AD flares could not be controlled with PIM.
Since there is a difference in the pathophysiology of AD between Asian and European population, a sub-group analysis of the pivotal PETITE study was conducted to provide a better insight toward the efficacy and safety of PIM in Chinese population. Compared to the PETITE study, the Chinese sub-group population was slightly younger, with more male patients enrolled, TBSA affected was larger and more patients had a total or facial IGA of 3. The mean exposure to PIM (in number of days) was lower in the Chinese population compared with the overall study population (207.9 vs 328.6 days). Also, the mean number of days of TCS use in the Chinese population was about 50% lower than in overall study population (28.8 vs 56.5 days) whereas the TCS exposure in the TCS only group was higher (354.4 vs 293.3 days). In the Chinese population a higher number of patients in the PIM did not use TCS compared to the overall study population (66.7 vs 41.9%); suggesting PIM had greater efficacy in the Chinese population (Citation11).
The adverse events observed in the Chinese subgroup (89.1%) was comparable in type and frequency with the overall study population (96.0%). In the PETITE study, the most frequent AEs in the PIM group was bronchitis, infected eczema, impetigo, and nasopharyngitis. In case of the Chinese subgroup, the main AEs experienced were diarrhea, pyrexia, nasopharyngitis, upper respiratory tract infection and cough ().
The treatment success for facial IGA progressively increased in the Chinese population similar to that of the overall study. At year 5, the Chinese population showed mild increase in overall IGA treatment success compared with the overall study (89.7% vs 88.7%). Similarly, progressive increase in facial IGA treatment success in the Chinese population was observed similar to the overall study. By week 3, median TBSA affected by AD decreased from 19% at baseline to 5% in PIM and 4% in TCS. In the global PETITE study, the median TBSA decreased from 16% to 3.8% in PIM and 4% in TCS by week 3. The decrease in median TBSA was comparable for the Chinese subgroup and the global PETITE study. However, it is worth noting that the baseline TBSA percentage was higher in the Chinese subgroup compared with the global study (19% vs 16%). The median TBSA affected by AD decreased to 0% after 1.5 years of ‘as-needed’ treatment and was maintained at this level for the rest of the study which was similar to the global PETITE study.
The baseline IGA and TBSA were higher in case of the PIM group (IGA = 3, 65% vs 49.2%[PIM vs TCS], TBSA 27.5% vs 26.7%[PIM vs TCS]). Hence, it took greater time for the PIM group to reach 0% TBSA. Recent studies evaluating safety of PIM in children and infants did not find any risk of malignancy with long term usage of PIM cream (Citation26,Citation27). A cohort study among 66,176 Asian patients was done to evaluate the association between TCIs and cancer among patients with atopic and endogenous eczema. Analysis showed that PIM was safe and was not associated with risk of cancer among pediatric patients (Citation28). The limitation of the study is that there was a high drop-out rate in the PIM group (50%). However, the main reason for drop-out was consent withdrawal rather than any serious AE. The study was conducted between 2004 and 2005 when the FDA had introduced the black box warning for PIM. This might have contributed toward the consent withdrawal. No malignancies or benign neoplasm were reported in the patients who had completed the study.
5. Conclusions
PIM showed an early and sustained efficacy in the Chinese sub-population affected with AD with a substantial corticosteroid-sparing effect. Overall, PIM is well tolerated in the Chinese sub-population, showing that it is safe for use in infants and young children. AEs were reported by similar proportions of patients in both PIM and TCS groups. However, the numbers of SAEs were lower in the PIM group than in the TCS group. The AEs observed in the Chinese study population were comparable in type and frequency with the overall study population.
Author contributions
All the authors were involved in the planning, execution, and reporting of the study. The manuscript was reviewed by all the authors and consent was given for the publication of the manuscript.
Acknowledgements
The authors appreciate the editorial support from Arghya Bhattacharya, PhD of Viatris.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Raw data were generated at Viatris. Derived data supporting the findings of this study are available from the corresponding author X.D. on request.
Additional information
Funding
References
- Kowalska-Olędzka E, Czarnecka M, Baran A. Epidemiology of atopic dermatitis in Europe. J Drug Assess. 2019;8(1):1–7. doi: 10.1080/21556660.2019.1619570.
- Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12(1):52. doi: 10.1186/s13223-016-0158-5.
- Guo Y, Li P, Tang J, et al. Prevalence of atopic dermatitis in Chinese children aged 1-7 ys. Sci Rep. 2016;6(1):29751. doi: 10.1038/srep29751.
- Wang HL, Sun J, Qian ZM, et al. Association between air pollution and atopic dermatitis in Guangzhou, China: modification by age and season. Br J Dermatol. 2021;184(6):1068–1076. doi: 10.1111/bjd.19645.
- Luk D, Hon KLE, Dizon MVC, et al. Practical recommendations for the topical treatment of atopic dermatitis in South and East Asia. Dermatol Ther. 2021;11(1):275–291. doi: 10.1007/s13555-020-00467-8.
- Stalder JF, Aubert H, Anthoine E, et al. Topical corticosteroid phobia in atopic dermatitis: international feasibility study of the TOPICOP score. Allergy. 2017;72(11):1713–1719. doi: 10.1111/all.13189.
- Luger T, Augustin M, Lambert J, et al. Unmet medical needs in the treatment of atopic dermatitis in infants: an expert consensus on safety and efficacy of Pimecrolimus. Pediatr Allergy Immunol. 2021;32(3):414–424. doi: 10.1111/pai.13422.
- Hanna S, Zip C, Shear NH. What is the risk of harm associated with topical calcineurin inhibitors? J Cutan Med Surg. 2019;23(4_suppl):19S–26S. doi: 10.1177/1203475419857688.
- Devasenapathy N, Chu A, Wong M, et al. Cancer risk with topical calcineurin inhibitors, Pimecrolimus and tacrolimus, for atopic dermatitis: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7(1):13–25. doi: 10.1016/S2352-4642(22)00283-8.
- Yao X, Song Z-Q, Li W, et al. Guidelines for diagnosis and treatment of atopic dermatitis in China (2020)#. Int J Dermatol Venereol. 2021;4(1):1–9. doi: 10.1097/JD9.0000000000000143.
- Sigurgeirsson B, Boznanski A, Todd G, et al. Safety and efficacy of Pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. 2015;135(4):597–606. doi: 10.1542/peds.2014-1990.
- Staab D, Pariser D, Gottlieb AB, et al. Low systemic absorption and good tolerability of Pimecrolimus, administered as 1% cream (Elidel) in infants with atopic dermatitis–a multicenter, 3-week, open-label study. Pediatr Dermatol. 2005;22(5):465–471. doi: 10.1111/j.1525-1470.2005.00128.x.
- Kaufmann R, Folster-Holst R, Hoger P, et al. Onset of action of Pimecrolimus cream 1% in the treatment of atopic eczema in infants. J Allergy Clin Immunol. 2004;114(5):1183–1188. doi: 10.1016/j.jaci.2004.08.015.
- Ho VC, Gupta A, Kaufmann R, et al. Safety and efficacy of nonsteroid Pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. J Pediatr. 2003;142(2):155–162. doi: 10.1067/mpd.2003.65.
- Kapp A, Papp K, Bingham A, et al. Long-term management of atopic dermatitis in infants with topical Pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol. 2002;110(2):277–284. doi: 10.1067/mai.2002.126500.
- Papp KA, Werfel T, Folster-Holst R, et al. Long-term control of atopic dermatitis with Pimecrolimus cream 1% in infants and young children: a two-year study. J Am Acad Dermatol. 2005;52(2):240–246. doi: 10.1016/j.jaad.2004.09.016.
- Paul C, Cork M, Rossi AB, et al. Safety and tolerability of 1% Pimecrolimus cream among infants: experience with 1133 patients treated for up to 2 years. Pediatrics. 2006;117(1):e118–e128. doi: 10.1542/peds.2005-1188.
- Zhao Z, Gao XH, Li W, et al. Experts’ consensus on the use of Pimecrolimus in atopic dermatitis in China: a TCS-sparing practical approach. Dermatol Ther. 2022;12(4):933–947. doi: 10.1007/s13555-022-00696-z.
- Noda S, Suarez-Farinas M, Ungar B, et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136(5):1254–1264. doi: 10.1016/j.jaci.2015.08.015.
- Chan TC, Sanyal RD, Pavel AB, et al. Atopic dermatitis in Chinese patients shows TH2/TH17 skewing with psoriasiform features. J Allergy Clin Immunol. 2018;142(3):1013–1017. doi: 10.1016/j.jaci.2018.06.016.
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143(1):1–11. doi: 10.1016/j.jaci.2018.10.032.
- Chu CY, Yao TC, Shih IH, et al. Pimecrolimus for the treatment of atopic dermatitis in infants: an Asian perspective. Dermatol Ther. 2023;13(3):717–727. doi: 10.1007/s13555-022-00886-9.
- Seymour JL, Keswick BH, Hanifin JM, et al. Clinical effects of diaper types on the skin of normal infants and infants with atopic dermatitis. J Am Acad Dermatol. 1987;17(6):988–997. doi: 10.1016/s0190-9622(87)70288-6.
- Berardesca E, Farage M, Maibach H. Sensitive skin: an overview. Int J Cosmet Sci. 2013;35(1):2–8. doi: 10.1111/j.1468-2494.2012.00754.x.
- Cheng J, Wu JJ, Han G. Epidemiology and characterization of atopic dermatitis in East Asian populations: a systematic review. Dermatol Ther. 2021;11(3):707–717. doi: 10.1007/s13555-021-00516-w.
- Arana A, Pottegard A, Kuiper JG, et al. Long-term risk of skin cancer and lymphoma in users of topical tacrolimus and Pimecrolimus: final results from the extension of the cohort study Protopic Joint European Longitudinal Lymphoma and Skin Cancer Evaluation (JOELLE). Clin Epidemiol. 2021;13:1141–1153. doi: 10.2147/CLEP.S331287.
- Paller AS, Folster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83(2):375–381. doi: 10.1016/j.jaad.2020.03.075.
- Cai SC, Li W, Tian EA, et al. Topical calcineurin inhibitors in eczema and cancer association: a cohort study. J Dermatolog Treat. 2016;27(6):531–537. doi: 10.3109/09546634.2016.1163317.