Abstract
Background and objective
Real-world evidence on persistence of interleukin-17 inhibitors (IL-17i) as a drug class among Japanese patients with psoriasis is lacking. Hence, we aimed to describe persistence rates of IL-17is among patients with psoriasis including psoriasis vulgaris (PsO), psoriatic arthritis (PsA), and generalized pustular psoriasis (GPP) or erythrodermic psoriasis (EP) in Japan.
Methods
We analyzed claims data from the Medical Data Vision database. Patients ≥15 years old with a psoriasis diagnosis and an IL-17i prescription between November 2016 and August 2020 were included and followed through August 2021. Persistence rates of the IL-17i class among patients with psoriasis and its subtypes (PsO, PsA, and GPP or EP), and persistence rates of ixekizumab, secukinumab, or brodalumab among patients with PsO or PsA were analyzed using Kaplan-Meier method. Analyses were conducted in the bio-naïve and bio-experienced subgroups.
Results
The IL-17i class had >50% persistence rates up to 36 months among patients with psoriasis and its subtypes (PsO, PsA, and GPP or EP). 36-Month persistence rates for ixekizumab, secukinumab, and brodalumab were 46.2% to 57.7% in patients with PsO and 43.0% to 48.4% in patients with PsA. Across analyses, bio-naïve patients demonstrated similar or greater persistence rates than bio-experienced patients.
Conclusion
IL-17is’ persistence rates over 36 months were >50% among patients with psoriasis and its subtypes (PsO, PsA, and GPP or EP) in Japan.
© 2023 eli lilly Japan K.K
Introduction
Psoriasis is a chronic inflammatory and remitting autoimmune disease affecting the skin; it may also manifest in the joints and nails (Citation1). The prevalence of psoriasis in Japan is estimated to be 0.34%, affecting >429,000 individuals (Citation2), predominantly males (Citation3). Four types of psoriasis are diagnosed during clinical practice in Japan—psoriasis vulgaris (PsO), generalized pustular psoriasis (GPP), erythrodermic psoriasis (EP), and psoriatic arthritis (PsA) (Citation4).
Mild psoriasis is managed with topical therapy, whereas moderate-to-severe psoriasis requires phototherapy or systemic therapy. The latter includes small molecules such as methotrexate, cyclosporine, and apremilast as well as biologics (Citation5). As of November 2022, 12 biologics have been approved for the treatment of psoriasis in Japan (Supplementary Material Appendix 1) (Citation6–8). In Japan, among the interleukin-17 inhibitors (IL-17is), ixekizumab and brodalumab have been approved for the treatment of all the commonly observed psoriasis subtypes (PsO, GPP, EP, and PsA) (Citation9); secukinumab has been approved for the treatment of PsO, GPP, and PsA (Citation10); and bimekizumab has been approved for the treatment of PsO, GPP, and EP (Citation7).
Despite the presence of IL-17is and other treatments, suboptimal persistence to therapy may lead to treatment failure and adversely impact economic management in healthcare systems (Citation11). Therefore, evaluating and improving medication-taking behavior of patients with psoriasis may enhance treatment strategy and limit economic expenditure.
In real-world studies from the United States (US), ixekizumab has demonstrated greater persistence versus secukinumab for up to 3 years of follow-up (Citation12,Citation13), and versus other IL-17is (secukinumab and brodalumab) for up to 2 years of follow-up (Citation14). In Japan, mixed evidence on persistence of interleukin-12/23 p40 subunit inhibitors (IL-12/23 p40i) (Citation11,Citation15–17) and interleukin-23 p19 subunit inhibitors (IL-23 p19i) (Citation11) versus IL-17is exists. However, only one Japanese single-center study by Ohata et al. (Citation18) compared IL-17is among 66 patients with psoriasis and reported no significant differences in persistence. Moreover, Ohata et al. could not evaluate persistence among different psoriasis patient groups due to limited sample size. Therefore, evidence from a real-world database study on persistence of IL-17is as a drug class among Japanese patients with psoriasis is still lacking. To address this knowledge gap, we evaluated real-world administrative claims data to understand the persistence rates of IL-17i biologics among patients with psoriasis and its subtypes (PsO, PsA, and GPP or EP) in Japan. We grouped GPP and EP together since these subtypes are rare and hard-to-treat.
Methods
Data source and study design
We used anonymized data from the Japanese Medical Data Vision (MDV) database. Since April 2008, the MDV database has been collecting deidentified inpatient and outpatient administrative claims and Diagnosis Procedure Combination (DPC) data from acute care hospitals and other services in Japan. The DPC is a case-mix classification system and is linked to a flat-fee payment system (Citation19,Citation20). As of August 2021 (the end of the current study), the MDV database covered approximately 38 million patients from 460 acute care hospitals (approximately 26% of all acute care Japanese hospitals).
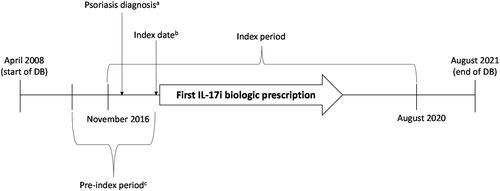
This retrospective observational study included MDV data from April 2008 to August 2021 (study period; ). Index identification period was from 1 November 2016 through 31 August 2020. We chose 1 November 2016 as the start of the index period because ixekizumab was launched in November 2016 in Japan for the treatment of psoriasis and its subtypes (PsO, PsA, GPP, and EP) (Citation9). Hence, three IL-17i class drugs were available in November 2016—ixekizumab, secukinumab, and brodalumab. We chose 31 August 2020 as the end of the index period to allow at least 1 year of follow-up (till August 2021). Index date was defined as the date of the first IL-17i class drug prescription (ixekizumab, secukinumab, or brodalumab) after the first psoriasis diagnosis in the index period. Pre-index period was the period from 6 months before the index date.
Patient population
The study included patients who were ≥15 years old (consistent with the approved label) at index date, had ≥1 claim for an IL-17i drug on or after the first psoriasis diagnosis in the index period, and were continuously enrolled in the database for at least 6 months before the index date and 1 year after the index date. Bimekizumab was not included in the current study as it was approved for use in Japan after the study period. Eligible patients were identified during the index period using the International Classification of Diseases, Tenth Revision (ICD-10) World Health Organization diagnosis codes—psoriasis (L40.0, L40.1, L40.5, L40.8, L40.9, M07.0–M07.3); PsO (L40.0 or L40.9); GPP or EP (L40.1 or L40.8); and PsA (L40.5 or M07.0–M07.3).
Patients using secukinumab or brodalumab were excluded if the following three criteria were met—their pre-index period began before 1 November 2016; they took secukinumab or brodalumab between the start of the pre-index period and 1 November 2016; and they did not switch to other IL-17i drugs but continued the same treatment after 1 November 2016. Patients diagnosed with PsO were excluded if they had PsA, GPP, or EP as comorbidities within 6 months before the index date. Patients diagnosed with GPP, EP, or PsA were excluded if they had a PsO diagnosis code before diagnosis of GPP, EP, or PsA; and if they took ixekizumab, secukinumab, or brodalumab in the pre-index period, but continued the same treatment (including generics). Patients with a diagnosis of ankylosing spondylitis (ICD-10 Code M45) or nonradiographic axial spondyloarthritis (ICD-10 Code M46.8) during the pre-index period were excluded.
Objectives and variables
The primary objective of the study was to assess persistence rate of IL-17i class among patients with PsO only. The secondary objectives were to describe the demographic and clinical characteristics of patients with psoriasis who received IL-17i class drug prescriptions, and to separately assess persistence rate of IL-17i class among patients with psoriasis, GPP or EP, or PsA. We also performed separate exploratory analyses for patients with PsO or PsA to understand the persistence rates of individual drugs in the IL-17i class (ixekizumab, secukinumab, or brodalumab). Persistence rates were reported for up to 36 months. All analyses among patients with PsO or PsA were stratified by bio-naïve or bio-experienced status. Patients were considered as bio-experienced if they had used any biologic drug specified in Supplementary Material Appendix 1—except bimekizumab or spesolimab—before the index date. Patients were considered as bio-naïve if they had not used any biologic drug before the index date.
Persistence was defined as being on continuous treatment from the time of initiation (index date) till the last day of supply of the index drug, allowing for a maximum gap of 60 days between the last day of supply of the prior prescription and the next prescription date. Treatments were considered to be discontinued when the gap was >60 days. Patients were considered to have reinitiated the index drug if there was ≥1 claim of the index drug after the discontinuation date. Reinitiation was measured only among the patients who discontinued the index drug. Patients were considered to have switched treatments when the index drug was discontinued, and another biologic was prescribed. If a patient switched to another drug within the IL-17i drug class, a second index date was considered for the new drug. Thus, the patient could be included in different treatment groups. If a patient discontinued the index drug and then reinitiated use of the index drug, only the date of the first prescription of the index drug was considered as the index date. Patients were considered to be on combined therapy if the index drug use overlapped with use of apremilast, cyclosporine, or methotrexate for ≥30 days. The number and proportion of patients who discontinued, reinitiated, switched, or used combined therapy were reported.
We also assessed the patients’ demographic and clinical characteristics. Clinical characteristics included any biologic, topical, or oral therapies, or phototherapy received during the pre-index period; and Charlson Comorbidity Index (CCI) (Citation21). CCI is a method of categorizing patient comorbidities based on ICD codes. A score of 0 indicates a lack of comorbidities, whereas higher scores indicate a greater likelihood of the predicted outcome resulting in mortality or higher healthcare resource use (Citation21). CCI was evaluated using claims data reported during the pre-index period. Comorbidities were described as individual conditions (yes/no) for each patient, and the number of conditions was recorded.
Statistical analysis
Demographic and clinical characteristics were evaluated descriptively. Categorical variables were presented as counts and percentages, and continuous variables were presented as means and standard deviations (SD). Kaplan–Meier method was used to assess persistence rates up to 36 months and 95% confidence intervals were estimated with log-log transformation. Adjustments by covariates were not performed. Patients who continued treatment with index drugs were censored at the earliest date among death, last observation of the database, or end of the study. For patients with PsO only, an additional censoring event was the development of GPP, EP, or PsA during the follow-up period. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used to perform the analyses.
Results
After applying the selection criteria, a total of 1686 patients with psoriasis were identified. Among them, 967, 570, and 207 patients were included in the PsO, PsA, and GPP or EP subtypes, respectively (). The number of patients treated with ixekizumab, secukinumab, and brodalumab varied for each psoriasis subtype ().
Figure 2. Selection of the study population.
Patients using SEC or BRO were excluded if the following three criteria were met – their pre-index period began before 1 November 2016; they experienced SEC or BRO between the start of the pre-index period and 1 November 2016; and they did not switch to other IL-17i drugs but continued the same treatment after 1 November 2016. Patients diagnosed with PsO were excluded if they had PsA, GPP, or EP as comorbidities within 6 months before the index date. Patients diagnosed with GPP, EP, or PsA were excluded if they had a PsO diagnosis code before diagnosis of GPP, EP, or PsA; and experienced IXE, SEC, or BRO in the pre-index period, but continued the same treatment (including generics). Patients with a diagnosis of ankylosing spondylitis (ICD-10 code M45) or non-radiographic axial spondyloarthritis (ICD-10 code M46.8) during the pre-index period were excluded.
BRO: brodalumab; EP: erythrodermic psoriasis; GPP: generalized pustular psoriasis; ICD-10: International Classification of Diseases, Tenth Revision; IL-17i: interleukin-17 inhibitors; IXE: ixekizumab; Ps: psoriasis; PsA: psoriatic arthritis; PsO: psoriasis vulgaris; SEC: secukinumab; WHO: World Health Organization.
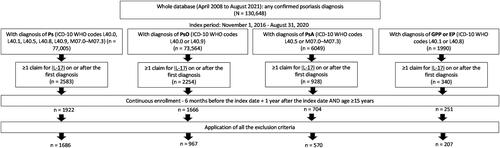
Table 1. Number of patients on IL-17 inhibitors in each cohort.
Patient characteristics and treatment outcomes of IL-17i class drug users
The mean age of the patients was similar (55 to 57 years) across the disease cohorts, whereas the proportion of males ranged from 49.3% in the GPP or EP cohort to 72.8% in the PsO cohort. Most patients were treated in large-scale hospitals (≥500 beds; >59.3%). Mean (SD) CCI ranged from 0.6 (1.1) in the PsO cohort to 1.3 (1.2) in the PsA cohort and 1.3 (1.4) in the GPP or EP cohort. Topical therapy was used the most across cohorts (≥54.0%), followed by oral therapy (≥32.7%). The proportion of bio-experienced patients ranged from 19.5% in the PsO cohort to 38.4% in the PsA cohort. IL-12/23 p40i were used the most in patients with PsO (9.7%), whereas tumor necrosis factor (TNF)-α inhibitors use was highest in all the other cohorts ().
Table 2. Patient characteristics for IL-17 inhibitor class drug users.
Across cohorts, the proportion of patients who discontinued biologics ranged from 21.8% to 26.0%, those who switched biologics ranged from 11.9% to 20.5%, those who reinitiated biologics ranged from 21.1% to 26.1%, and those on combination therapy with apremilast, cyclosporine, or methotrexate for ≥30 days ranged from 12.1% to 33.3% ().
Table 3. Treatment outcomes for IL-17 inhibitor class drug users till the end of the follow-up period.
IL-17i treatment persistence rates among patients with psoriasis, PsO, PsA, or GPP or EP
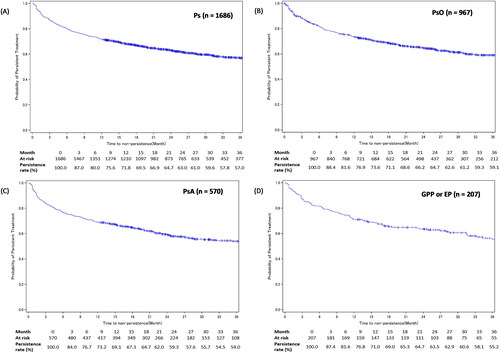
The IL-17i treatment persistence rates at 12, 24, and 36 months, respectively, from treatment initiation were 71.8%, 63.0%, and 57.0% among patients with psoriasis (); 73.6%, 64.7%, and 59.1% among patients with PsO (); 69.1%, 59.3%, and 54.0% among patients with PsA (); and 71.0%, 63.5%, and 55.2% among patients with GPP or EP ().
Figure 3. IL-17i treatment persistence among patients with Ps, PsO, PsA, or GPP or EP.
Figures A-D show the Kaplan-Meier curves for persistence of IL-17i class drugs in patients with Ps, PsO, PsA, or GPP or EP, respectively.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
EP: erythrodermic psoriasis; GPP: generalized pustular psoriasis; IL-17i: interleukin-17 inhibitor; Ps: psoriasis; PsA: psoriatic arthritis; PsO: psoriasis vulgaris.
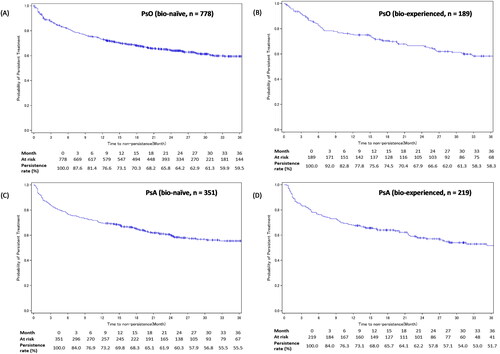
Subgroup analysis by experience with biologics showed similar persistence rates among bio-naïve and bio-experienced patients with PsO at 12 (73.1% vs 75.6%), 24 (64.2% vs 66.6%), and 36 months (59.5% vs 58.3%) (); as well as among bio-naïve and bio-experienced patients with PsA at 12 (69.8% vs 68.0%), 24 (60.3% vs 57.8%), and 36 months (55.5% vs 51.7%) ().
Figure 4. IL-17i treatment persistence among patients with PsO or PsA – stratified by experience with biologics.
Figures A-B show the Kaplan-Meier curves for persistence of IL-17i class drugs in bio-naïve and bio-experienced patients with PsO. Figures C-D show the Kaplan-Meier curves for persistence of IL-17i class drugs in bio-naïve and bio-experienced patients with PsA.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
IL-17i: interleukin-17 inhibitor; PsA: psoriatic arthritis; PsO: psoriasis vulgaris.
Patient characteristics and treatment outcomes of PsO-diagnosed ixekizumab, secukinumab or brodalumab users
The mean age of the patients (54 to 56 years) and proportion of males (71% to 75%) was similar across the PsO-diagnosed ixekizumab, secukinumab, and brodalumab cohorts. Most patients were treated in large-scale hospitals (≥500 beds; ≥63.0%). Mean CCI was similar across the cohorts. Topical therapy was used the most across cohorts (≥65.8%). Prior use of biologics was highest in brodalumab users (38.5%) followed by ixekizumab users (32.9%), and secukinumab users (21.9%). Prior use of IL-17i was highest in brodalumab users (22.1%), while prior use of IL-12/23 p40i was highest in secukinumab users (10.0%) ().
Table 4. Patient characteristics of PsO-diagnosed IXE, SEC, or BRO users.
Discontinuation of biologics was highest in PsO-diagnosed secukinumab users (29.0%) and lowest in brodalumab users (19.2%). Switching of biologics was similar across cohorts (17.7% to 19.9%). Ixekizumab users most frequently switched to guselkumab (4.7%) or brodalumab (4.3%), secukinumab users most frequently switched to brodalumab (5.1%) or guselkumab (4.3%), and brodalumab users most frequently switched to ixekizumab (6.3%) or guselkumab (5.7%). Reinitiation of biologics occurred the most in secukinumab users (24.1%). Use of combination therapy with apremilast, cyclosporin, or methotrexate for ≥30 days was similar across cohorts ().
Table 5. Treatment outcomes for PsO-diagnosed IXE, SEC, or BRO users.
Treatment persistence rates of ixekizumab, secukinumab or brodalumab in patients with PsO
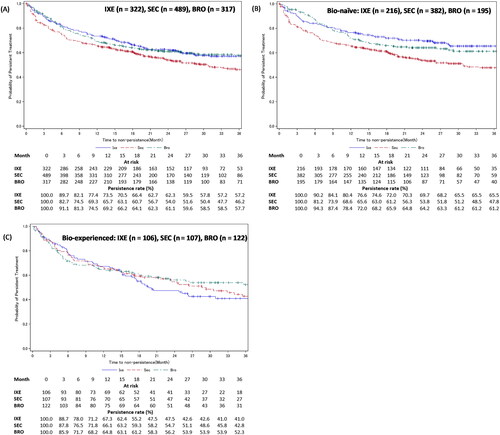
In patients with PsO, the treatment persistence rates at 12, 24, and 36 months, respectively, from treatment initiation were 73.5%, 62.3%, and 57.2% for ixekizumab, 65.7%, 54.0%, and 46.2% for secukinumab, and 69.2%, 61.1%, and 57.7% for brodalumab (). Treatment persistence was also stratified based on experience with biologics. For ixekizumab, bio-naïve patients had numerically higher persistence rates than bio-experienced patients at 12 (76.6% vs 67.3%), 24 (69.7% vs 47.5%), and 36 months (65.5% vs 41.0%) (). For brodalumab, bio-naïve patients had numerically higher persistence rates than bio-experienced patients at 12 (72.0% vs 64.8%), 24 (64.2% vs 56.2%), and 36 months (61.2% vs 52.3%) (). For secukinumab, bio-naïve and bio-experienced patients had similar persistence rates at 12 (65.6% vs 66.1%), 24 (53.8% vs 54.7%), and 36 months (47.8% vs 42.8%) ().
Figure 5. Treatment persistence of IXE, SEC, or BRO in patients with PsO – stratified by experience with biologics.
Figure A shows the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in patients with PsO. Figures B and C show the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in bio-naïve and bio-experienced patients with PsO.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
BRO: brodalumab; IXE: ixekizumab; PsO: psoriasis vulgaris; SEC: secukinumab.
Patient characteristics and treatment outcomes of PsA-diagnosed ixekizumab, secukinumab or brodalumab users
The mean age of the patients was similar across PsA-diagnosed ixekizumab, secukinumab, and brodalumab cohorts (53 to 56 years) whereas the proportion of males ranged from 51.0% in the secukinumab cohort to 66.0% in the brodalumab cohort. Most patients were treated in large-scale hospitals (≥500 beds; ≥56.4%). Mean CCI was similar across the cohorts. Use of topical therapy varied from 49.6% in the secukinumab cohort to 71.8% in the brodalumab cohort. Prior use of biologics was highest in brodalumab users (65.0%) followed by ixekizumab (48.7%), and secukinumab users (40.9%). Prior use of TNF-α was highest in secukinumab users (27.9%), whereas prior use of IL-17is was highest in brodalumab users (37.9%) ().
Table 6. Patient characteristics of PsA-diagnosed IXE, SEC, or BRO users.
Discontinuation of biologics was highest in PsA-diagnosed secukinumab users (22.6%) and lowest in ixekizumab users (18.2%). Switching of biologics was highest in brodalumab users (33.0%) and lowest in secukinumab users (26.7%). Ixekizumab users most frequently switched to guselkumab (8.1%) or adalimumab (7.6%), SEC users most frequently switched to adalimumab (8.6%), and brodalumab users most frequently switched to ixekizumab (10.7%). Reinitiation of biologics occurred the most in secukinumab users (19.0%). Use of combination therapy with apremilast, cyclosporine, or methotrexate for ≥30 days was similar across cohorts ().
Table 7. Treatment outcomes for PsA-diagnosed IXE, SEC, or BRO users.
Treatment persistence rates of ixekizumab, secukinumab or brodalumab in patients with PsA
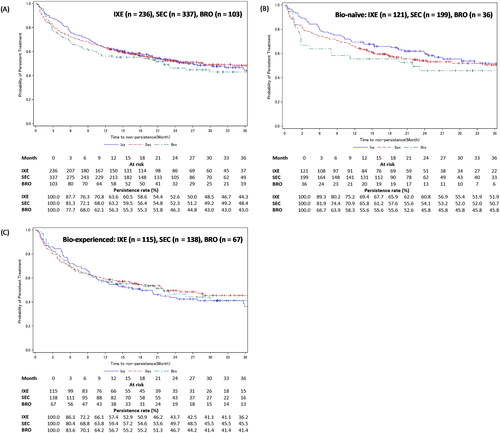
In patients with PsA, the treatment persistence rates at 12, 24, and 36 months, respectively, from treatment initiation were 63.6%, 52.6%, and 44.3% for ixekizumab, 63.2%, 52.3%, and 48.4% for secukinumab, and 56.3%, 46.3%, and 43.0% for brodalumab (). Treatment persistence was also stratified based on experience with biologics. For ixekizumab, bio-naïve patients had numerically higher persistence rates than bio-experienced patients at 12 (69.4% vs 57.4%), 24 (60.8% vs 43.7%), and 36 months (51.9% vs 36.2%) (). For secukinumab, bio-naïve patients had numerically higher persistence rates than bio-experienced patients at 12 (65.8% vs 59.4%), 24 (54.1% vs 49.7%), and 36 months (50.7% vs 45.5%) (). For brodalumab, bio-naïve and bio-experienced patients had similar persistence rates at 12 (55.6% vs 56.7%), 24 (45.8% vs 46.7%), and 36 months (45.8% vs 41.4%) ().
Figure 6. Treatment persistence of IXE, SEC, or BRO in patients with PsA – stratified by experience with biologics.
Figure A shows the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in patients with PsA. Figures B and C show the Kaplan-Meier curves for persistence of IXE, SEC, and BRO in bio-naïve and bio-experienced patients with PsA.
Development of GPP, EP, or PsA in the PsO cohort’s patients was considered as an additional censoring event.
BRO: brodalumab; IXE: ixekizumab; PsA: psoriatic arthritis; SEC: secukinumab.
Discussion
Biologics greatly improve the quality of life of patients with psoriasis (Citation22,Citation23). However, poor persistence to chronic biologic therapy may lead to suboptimal health outcomes (Citation11). Therefore, improving persistence on biologics is essential to ensure patients obtain optimum benefits as well as to reduce healthcare costs. This study describes the real-world treatment persistence rates of IL-17i class drugs among Japanese patients with psoriasis and its subtypes (PsO, PsA, and GPP or EP). As a drug class, patients on IL-17is had >50% persistence rate for up to 36 months after treatment initiation, irrespective of psoriasis subtype. The IL-17i drug class persistence rate was similar across psoriasis subtypes at every timepoint, with the highest persistence rate observed in patients with PsO (74% to 59%) and the lowest persistence rate in patients with PsA (69% to 54%). Individual drug persistence rates for ixekizumab, secukinumab, and brodalumab 36 months after treatment initiation ranged from 46% to 58% in patients with PsO and from 43% to 48% in patients with PsA. Across analyses, bio-naïve patients demonstrated similar or greater persistence rates than bio-experienced patients.
Other real-world studies have mostly evaluated the individual persistence rate of IL-17i drugs in patients with psoriasis or its subtypes; hence, evidence demonstrating the collective persistence rate of this drug class is rare. Therefore, we compared the persistence rate of the collective IL-17i drug class from our study with the persistence rate range of individual IL-17i drugs or persistence rate of the collective IL-17i drug class from other real-world studies. As the disease definitions used for including patients in real-world studies often differ, we compared our results with studies that used similar ICD codes.
Among patients with psoriasis, the 1-year persistence rate of IL-17is in our study (72%) was higher than that of studies from the US (50% to 67%) (Citation13,Citation24) but similar to a Japanese study (58%–73%) (Citation18). Among patients with PsO, the 1-year persistence rate of IL-17is in our study was lower than that observed in a French study (74% vs 81%) but the 2-year (65% vs 59%) and 3-year (59% vs 45%) rates were higher (Citation25). Among patients with PsA, the 1-year persistence rate of IL-17is in our study was lower than studies from Europe and the US (69% vs 73% to 80%) (Citation25–27). However, 2-year (59% vs 51%) and 3-year (54% vs 42%) persistence rates were higher than those observed in a French study (Citation25). The differences in persistence rates could be attributed to heterogeneity in study time periods, healthcare systems and associated practices, and study definitions.
Consistent with previous research (Citation11,Citation13,Citation14,Citation25,Citation28), in the current study, patients with PsO on ixekizumab and brodalumab demonstrated numerically greater persistence rates than secukinumab users over 36 months. However, among patients with PsA, ixekizumab and secukinumab showed numerically greater persistence rates than brodalumab. Although previous studies among patients with PsA have not compared the persistence rates of ixekizumab, secukinumab, and brodalumab, the similarity between the persistence rates of ixekizumab and secukinumab observed in the current study is in line with existing evidence (Citation24,Citation29,Citation30).
Biologics elicit the best response in biologic-naïve patients, and the response decreases with an increase in the number of prior biologic therapies (Citation31,Citation32). Real-world studies evaluating only IL-17is confirmed this tendency (Citation14,Citation28). In fact, Kojanova et al. (Citation28) reported 1.26 times greater risk of treatment discontinuation with every increase in the treatment line. We did not observe a large difference in the persistence rates of IL-17is as a drug class between bio-naïve and bio-experienced patients. However, comparing persistence rates of individual drugs among bio-naïve and bio-experienced patients showed notable differences. At 36 months, bio-naïve patients with PsO on ixekizumab, secukinumab, or brodalumab showed 5% to 25% greater persistence rate than bio-experienced patients, whereas bio-naïve patients with PsA on ixekizumab, secukinumab, or brodalumab showed 4% to 16% greater persistence rate than bio-experienced patients.
Persistence rates may decrease over time due to treatment failure, adverse reactions, patient requests, or other reasons. In such cases, physicians may discontinue treatment or switch patients to another biologic. In the current study, treatment switching was lowest among patients with PsO (12%) and highest among patients with PsA (21%). Patients with PsO were mostly switched to another IL-17i or an IL-23 p19i such as guselkumab, whereas patients with PsA were switched to another IL-17i, an IL-23 p19i, or the TNF-α inhibitor adalimumab. Physicians might have preferred switching to an IL-23 p19i, as the persistence rate of IL-23 p19i is similar to or greater than that of IL-17is among patients with PsO or PsA (Citation24,Citation27,Citation33–35).
Interruptions during biologic treatment may occur due to illness, hospitalization, pregnancy, or other factors, following which physicians may reinitiate biologic treatment. Wang et al. (Citation36) described how such interruptions in the biologic therapy of PsO followed by treatment reinitiation is safe and does not have a clinically significant impact. In the current study, 21% to 26% of patients across the psoriasis subtype cohorts reinitiated treatment. Although we did not clinically evaluate the impact of treatment reinitiation, such research from a real-world perspective could be conducted in the future to supplement evidence from clinical trials.
Limitations
There are a few limitations in this study. As the MDV database obtains data from the DPC system, we could include only patients treated within a DPC hospital. Patients could not be tracked if they did not return to the same DPC hospital or were transferred to another hospital or clinic outside the DPC system. Hence, these patients dropped out from the dataset at their final observation. Also, as the database cannot track a patient across multiple hospitals, patients might have been counted multiple times if they received treatment at >1 hospital within the DPC system. The MDV database includes only hospitals using the flat-fee payment system, that is, mostly large acute-phase hospitals; therefore, patients treated at small clinics are not captured. Furthermore, due to regulations imposed by the Japanese Dermatological Association, only accredited clinical facilities meeting pre-specified criteria (i.e., usually large hospitals) are allowed to administer biologics (Citation37). Such administration mostly occurs in the outpatient rather than the inpatient ward. Hence, the results of this study may not be generalizable to all patients with psoriasis, PsO, PsA, or GPP or EP in Japan. The study identified patients based on ICD-10 diagnostic codes present in the claims data. However, in routine clinical practice, some codes might have been inaccurately recorded resulting in misdiagnosis of a few cases. Therefore, the possibility of including misclassified patients cannot be ruled out. Some patients were followed up for a maximum of 1 year only, which might have affected the obtained results. Additionally, the persistence rate may have been affected since many patients refrained from visiting hospitals in April and May, 2020 when a state of emergency was declared due to the COVID-19 pandemic (Citation38). If patients developed PsA, GPP, or EP after diagnosis of PsO, they might have been included in multiple disease cohorts. Also, if patients switched treatments, they might have been included in >1 treatment cohort among ixekizumab, secukinumab, and brodalumab. GPP and EP have different pathological and clinical characteristics, so the use of biologics may vary. However, due to the rarity of these psoriasis subtypes we grouped them together for analysis. Finally, due to the nature of the database study, only limited variables could be referenced from the MDV database. Therefore, unmeasured confounding may have occurred, which could have affected the results, although the patient characteristics between ixekizumab, secukinumab, and brodalumab users were similar.
Conclusion
In this study the persistence rates of IL-17is were >50% up to 36 months from treatment initiation among patients with psoriasis, PsO, PsA, or GPP or EP in Japan. Our results may provide essential real-world evidence to help physicians understand treatment outcomes and long-term persistence rate data better, which is generally unavailable from clinical trials.
Ethics approval
This observational study used only data collected previously, does not include any intervention, and deidentifies the data to protect patient privacy. Therefore, a formal consent form is not required. As the study uses retrospective deidentified data, the Japanese Ethical Guidelines for Medical and Health Research do not apply, thus the study is exempt from ethical review. This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Pharmacoepidemiology Practices and applicable laws and regulations of Japan.
Author contributions
Conception and design: CW, HTI, TH, ZC, SO.
Analysis and data interpretation: CW, HTI, TH, TM, ZC, SO, TA.
Writing – critical revision: CW, HTI, TH, TM, ZC, SO, TA.
Supplemental Material
Download PDF (89.1 KB)Acknowledgments
Leo J. Philip Tharappel of Eli Lilly Services India Private Limited, Bengaluru, India provided medical writing and editorial support, which was funded by Eli Lilly Japan K.K.
Disclosure statement
CW, HTI, TH, TM, ZC, SO, and TA are employees of Eli Lilly Japan K.K. and hold stock and/or stock options of Eli Lilly and Company.
Data availability statement
The datasets generated and/or analyzed in the current study are not publicly available because they were commercially obtained from Medical Data Vision Co., Ltd., and were used under license. Data can be made available from Medical Data Vision Co., Ltd., upon request.
Additional information
Funding
References
- Tada Y, Ishii K, Kimura J, et al. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46(6):1–11. doi: 10.1111/1346-8138.14870.
- Kubota K, Kamijima Y, Sato T, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the japanese national claims database. BMJ Open. 2015;5(1):e006450. doi: 10.1136/bmjopen-2014-006450.
- Kamiya K, Oiso N, Kawada A, et al. Epidemiological survey of the psoriasis patients in the japanese society for psoriasis research from 2013 to 2018. J Dermatol. 2021;48(6):864–875. doi: 10.1111/1346-8138.15803.
- Ogawa E, Okuyama R, Seki T, et al. Epidemiological survey of patients with psoriasis in matsumoto city, Nagano prefecture, Japan. J Dermatol. 2018;45(3):314–317. doi: 10.1111/1346-8138.14101.
- Feldman SR. Treatment of psoriasis in adults: upToDate; 2022 updated 2022 Oct 12; [cited 2022 Nov 02]. Available from: https://www.uptodate.com/contents/treatment-of-psoriasis-in-adults#H45.
- List of Approved Products: Pharmaceuticals and Medical Devices Agency; [cited 2022 Nov 02]. Available from: https://www.pmda.go.jp/english/review-services/reviews/approved-information/drugs/0002.html.
- BIMZELX[®] (bimekizumab) Approved in Japan for the Treatment of Plaque Psoriasis, Generalized Pustular Psoriasis and Psoriatic Erythroderma: UCB. 2022; [cited 2022 Nov 28]. Available from: https://www.ucb.com/stories-media/Press-Releases/article/BIMZELXR-bimekizumab-Approved-in-Japan-for-the-Treatment-of-Plaque-Psoriasis-Generalized-Pustular-Psoriasis-and-Psoriatic-Erythroderma#:∼:text=Bimekizumab%20is%20the%20first%20approved,17A%20(IL%2D17A).
- Nippon Boehringer Ingelheim Receives Manufacturing and Marketing Approval for “Spevigo® Intravenous Infusion 450mg” for Treatment of Acute Pustular Psoriasis: Boehringer Ingelheim; [updated September 26, 2022; March 13, 2023]. Available from: https://www.boehringer-ingelheim.jp/press-release/20220926_01.
- New Drugs Approved in FY. 2016: Pharmaceuticals and Medical Devices Agency; [cited 2022 Nov 02]. Available from: https://www.pmda.go.jp/files/000232770.pdf.
- New Drugs Approved in FY. 2021: Pharmaceuticals and Medical Devices Agency; [cited 2022 Nov 02]. Available from: https://www.pmda.go.jp/files/000246734.pdf.
- Tada Y, Kim H, Spanopoulos D, et al. Treatment patterns, healthcare resource utilization, and costs in patients with moderate-to-severe psoriasis treated with systemic therapy in Japan: a retrospective claims database study. J Dermatol. 2022;49(11):1106–1117. doi: 10.1111/1346-8138.16543.
- Leonardi C, Zhu B, Malatestinic WN, et al. Real-world biologic adherence, persistence, and monotherapy comparisons in US patients with psoriasis: results from IBM MarketScan((R)) databases. Adv Ther. 2022;39(7):3214–3224. doi: 10.1007/s12325-022-02155-9.
- Blauvelt A, Shi N, Burge R, et al. Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab. J Am Acad Dermatol. 2020;82(4):927–935. doi: 10.1016/j.jaad.2019.11.015.
- Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona psoriasis registry. Dermatol Ther. 2021;34(2):e14808.
- Kishimoto M, Komine M, Kamiya K, et al. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J Dermatol. 2020;47(1):33–40. doi: 10.1111/1346-8138.15146.
- Bayaraa B, Imafuku S. Sustainability and switching of biologics for psoriasis and psoriatic arthritis at Fukuoka university psoriasis registry. J Dermatol. 2019;46(5):389–398. doi: 10.1111/1346-8138.14834.
- Sruamsiri R, Iwasaki K, Tang W, et al. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):5. doi: 10.1186/s12895-018-0074-0.
- Ohata C, Ohyama B, Katayama E, et al. Real-world efficacy and safety of interleukin-17 inhibitors for psoriasis: a single-center experience. J Dermatol. 2020;47(4):405–408. doi: 10.1111/1346-8138.15247.
- Matsuda S. Development of case mix based evaluation system in Japan. Jpn Hosp. 2016;(35):35–44.
- Hayashida K, Murakami G, Matsuda S, et al. History and profile of diagnosis procedure combination (DPC): development of a real data collection system for acute inpatient care in Japan. J Epidemiol. 2021;31(1):1–11. doi: 10.2188/jea.JE20200288.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83.
- Jungo P, Maul JT, Djamei V, et al. Superiority in quality of life improvement of biologics over conventional systemic drugs in a swiss Real-Life psoriasis registry. Dermatology. 2016;232(6):655–663. doi: 10.1159/000455042.
- Colombo D, Bianchi L, Fabbrocini G, et al. Real-world evidence of biologic treatments in moderate-severe psoriasis in Italy: results of the CANOVA (EffeCtiveness of biologic treAtmeNts for plaque psOriasis in Italy: an obserVAtional longitudinal study of real-life clinical practice) study. Dermatol Ther. 2022;35(1):e15166.
- Xu C, Teeple A, Wu B, et al. Treatment adherence and persistence of seven commonly prescribed biologics for moderate to severe psoriasis and psoriatic arthritis in a U.S. commercially insured population. J Dermatolog Treat. 2022;33(4):2270–2277. doi: 10.1080/09546634.2021.1950600.
- Pina Vegas L, Penso L, Claudepierre P, et al. Long-term persistence of first-line biologics for patients with psoriasis and psoriatic arthritis in the french health insurance database. JAMA Dermatol. 2022;158(5):513–522. doi: 10.1001/jamadermatol.2022.0364.
- Perrone V, Losi S, Filippi E, et al. Analysis of the pharmacoutilization of biological drugs in psoriatic arthritis patients: a real-world retrospective study among an Italian population. Rheumatol Ther. 2022;9(3):875–890. doi: 10.1007/s40744-022-00440-1.
- Walsh JA, Cai Q, Lin I, et al. Treatment persistence and adherence among patients with psoriatic arthritis who initiated targeted immune modulators in the US: a retrospective cohort study. Adv Ther. 2021;38(5):2353–2364. doi: 10.1007/s12325-021-01687-w.
- Kojanova M, Hugo J, Velackova B, et al. Efficacy, safety, and drug survival of patients with psoriasis treated with IL-17 inhibitors – brodalumab, ixekizumab, and secukinumab: real-world data from the Czech Republic BIOREP registry. J Dermatolog Treat. 2022;33(6):2827–2837. doi: 10.1080/09546634.2022.2082354.
- Egeberg A, Roseno NAL, Aagaard D, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis – a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53:151979. doi: 10.1016/j.semarthrit.2022.151979.
- Pizzicato LN, Vadhariya A, Birt J, et al. Real-world treatment patterns and use of adjunctive pain and anti-inflammatory medications among patients with psoriatic arthritis treated with IL-17A inhibitors in the United States. J Manag Care Spec Pharm. 2023;29(1):24–35.
- Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective Multi-Country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–579. doi: 10.1007/s40257-021-00598-4.
- Wade R, Sharif-Hurst S, Dias S. Patient characteristics as effect modifiers for psoriasis biologic treatment response: an assessment using network meta-analysis subgroups. Syst Rev. 2020;9(1):132. doi: 10.1186/s13643-020-01395-6.
- Yiu ZZN, Becher G, Kirby B, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158(10):1131–1141. doi: 10.1001/jamadermatol.2022.2909.
- Blauvelt A, Burge R, Gallo G, et al. A retrospective cohort analysis of treatment patterns over 1 year in patients with psoriasis treated with ixekizumab or guselkumab. Dermatol Ther . 2022;12(3):701–714. doi: 10.1007/s13555-022-00686-1.
- Xu C, Teeple A, Wu B, et al. Drug adherence and persistence of patients with moderate to severe psoriasis treated with biologic medications in a US commercially insured population. Dermatology. 2022;238(3):438–447. doi: 10.1159/000519176.
- Wang CY, Foley P, Baker C, et al. Biological therapy interruption and Re-treatment in chronic plaque psoriasis. J Drugs Dermatol. 2021;20(10):1063–1071.
- Saeki H, Mabuchi T, Asahina A, et. al. English version of Japanese guidance for use of biologics for psoriasis (the 2022 version). J Dermatol. 2023;50(2):e41–e68. doi: 10.1111/1346-8138.16691.
- Uchida H, Kamata M, Egawa S, et al. Impact of the COVID-19 pandemic on biologic treatment in psoriasis patients: a single-center retrospective study in Japan. J Dermatol. 2022;49(6):624–628. doi: 10.1111/1346-8138.16362.