Abstract
Aim
To emphasize the role of non-sulfonamides in the treatment of Nocardia infection and reduce the adverse reactions caused by sulfonamides.
Methods
We retrospectively analyzed a case of cutaneous nocardiosis in an immunocompetent individual. The colonies obtained by staining the pus in the lesion with antacid and culturing the agar plates were identified by flight mass spectrometry. The pathogenic identification showed Nocardia brasiliensis infection and the patient was treated with amoxicillin-clavulanic acid.
Results
After treatment with amoxicillin and clavulanic acid, the ulcer gradually peeled and crusted, leaving dark pigmentation. The patient has finally recovered.
Conclusion:
Sulfonamides are the first-line antibacterial agents for years in treatment of nocardiosis but are of great toxicity and side effects. This patient was successfully treated with amoxicillin-clavulanic acid and it provided a reference protocol for patients with sulfonamide-resistant Nocardia or sulfonamides intolerance.
1. Introduction
Trimethoprim-sulfamethoxazole is the first-line antibacterial agent for years in initial therapy of nocardiosis, occasionally combines with amikacin, third-generation cephalosporins, linezolid or imipenem (Citation1,Citation2). However, sulfonamide-resistant or linezolid-resistant Nocardia strains have been isolated in clinical in recent years, which brings great challenges to the clinical treatment of Nocardia (Citation3,Citation4). In addition, Sulfonamides are of great toxicity and side effects (Citation5–8), mainly including: (1) anaphylaxis, accompanied by skin itching, rash, dermatitis or angioneurotic edema; (2) jaundice, abnormal liver function, acute liver necrosis;(3) crystal deposition in the urine, causing hematuria and renal calculus; (4) granulopenia, thrombocytopenia, aplastic anemia; (5) sulfonamides enter the fetal circulation through the placenta and affect infant development. Therefore, non-sulfonamides treatment of nocardiosis should be emphasized. Here, we reported a case of cutaneous nocardiosis caused by Nocardia brasiliensis infection in an immunocompetent individual who was successfully treated with amoxicillin-clavulanic acid.
2. Case presentation
2.1. Clinical features
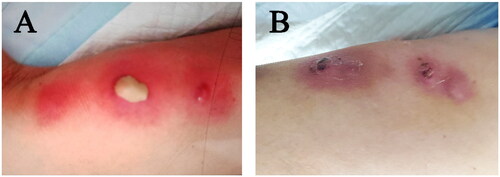
An 87-year-old female with a history of hypertension presented with multiple painful swelling erythematous lesions on her right lower limb two weeks ago. Skin examination revealed three pimples with white, stiff pustules on the top of the edematous plaques and right lower limb erythema, warmth and tenderness, the pimples began to fester and drain (). The patient had no history of trauma, no specific exposure.
2.2. Etiological examination
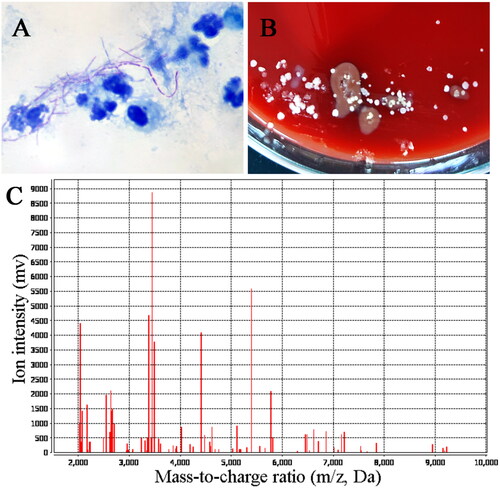
After the surface of the lesion was disinfected with iodophor, the pus in the focus was extracted for acid-fast staining, which showed weakly positive for branched rod-shaped bacteria (). Meanwhile, the pus was put into Columbia blood agar plates, chocolate agar plates and Candida chromogenic agar plates respectively in a carbon dioxide incubator for 24 h at 37 °C. After that, it was observed that there were different sizes, white, dry, and wrinkled protruding colonies on the blood plate (), which suggested that it might be nocardia infection. Finally, the bacterial colony was identified as Nocardia brasiliensis by flight mass spectrometry (). The Kirby–Bauer method was performed to determine the maximum zone of inhibition of the isolated Nocardia brasiliensis against amikacin, clarithromycin and an Epsilometer test was performed to determine the minimum inhibitory concentration value of the isolated strain against TMP-SMZ, Imipenem, Linezolid, levofloxacin and Amoxicillin-clavulanic acid ().
Figure 2. Pathogenic examination. (A) Acid fast staining showed it was weakly acid-fast with a filamentous appearance. (B) Bacterial colonies on rabbit blood agar after incubation at 37 °C for 24 h. (C) Matrix-assisted laser desorption/ionization-time of flight (MALTID-TOF) mass spectrometry showed it was Nocardia brasiliensis.
2.3. Medication and prognosis
After admission, the patient was given anti-infective treatment with piperacillin tazobactam 4.5 g intravenously, 3 times a day for 8 days. And debridement and surface disinfection of skin lesions. On day 8 of hospitalization, we changed the antibiotic to intravenous amoxicillin clavulanic acid 1.2 g twice a day for 5 days. The lesion gradually diminished in size, permitting discharge on the 13th hospital day, followed by continued oral amoxicillin-clavulanic acid 312.5 mg three times a day for another 8 days after discharge. The total duration of antibiotic treatment was 21 days. The ulcer gradually peeled and crusted, leaving dark pigmentation (). After discharge, we suggest that the patient come back to our hospital for reexamination one month and give rehabilitation training guidance. Within one week, the patient should have a proper rest, lower limb elevation, and a small amount of indoor activities. After one week, the patient can resume normal activities if there is no obvious discomfort. Avoid strenuous exercise for 2 weeks. Avoid standing for long periods of time and heavy physical work for one month.
3. Discussion
Nocardiosis is a suppurative or granulomatous inflammation caused by opportunistic Nocardia species (Citation9,Citation10). The most frequent clinical manifestation is pulmonary infection in immunodeficiency patients (Citation11). Cases of cutaneous nocardiosis in immunocompetent individuals are rare, and these patients are often misdiagnosed and delayed etiological treatment (Citation12). In this case, Nocardia was isolated subcutaneously from the patient who had no prior symptoms of immunodeficiency. According to the diagnostic guidelines (Citation13) and the clinical manifestations of patient, she was diagnosed as cutaneous nocardiosis.
Cutaneous nocardiosis can present in immunonormal individuals in three different forms, including actinomycoma, superficial skin infection, and lymphatic skin infection (Citation14). The case we described belongs to superficial skin infection and it’ s main clinical manifestations are pustules, ulcers, granulomas, abscesses or cellulitis (Citation15–17). The patient presented with multiple painful redness and swelling in the right lower limb with white hard pustules above the edematous plaque. Radiographic and laboratory findings of the patient showed no indication of disseminated infection. We successfully cured the patient through the combination of amoxicillin-clavulanic acid.
Different Nocardia species showed different susceptibilities to various antimicrobial agents. In general, TMP-SMX is the first line of treatment for Nocardia infection, and 90% to 100% of Nocardia are sensitive to TMP-SMX (Citation3,Citation18). However, TMP-SMX, as a sulfonamide drug, has large toxic and side effects to the host (Citation19). In addition, linezolid, imipenem and ceftriaxone are often used in combination with TMP-SMX to treat Nocardia infection. Most Nocardia showed high sensitivity to linezolid (Citation20), but the long-term use of linezolid can cause blood toxicity and nerve damage (Citation21). Moreover, Nocardia has strong resistance to imipenem and ceftriaxone. According to a recent study in Australia, the resistance rate of Nocardia to imipenem and ceftriaxone is as high as 49% and 42% (Citation22). Hamdi et al. showed that 74% of Nocardia. Transvalensis complex and 38% of Nocardia pseudobrasiliensis were resistant to mikacin, 96% of Nocardia. Nova complex and 92% of Nocardia cyriacigeorgica were resistant to amoxicillin clavulanic acid (Citation9). Therefore, the treatment of Nocardia depends on the identification of the strain and the results of drug sensitivity.
The pathogenic bacteria were identified by MALDI-TOF MS with 99.9% confidence value as Nocardia brasiliensis. It was reported that Nocardia brasiliensis had a high sensitivity to amoxicillin-clavulanic acid while other Nocardia had a low sensitivity to amoxicillin-clavulanic acid (Citation23). Although we hadn’t identified the pathogenic bacteria at the beginning of treatment, we gave patient intravenous piperacillin-tazobactam anti-infective therapy empirically. Then the pathogenic examination showed that it was caused by Nocardia brasiliensis, we changed the treatment mode and used intravenous amoxicillin clavulanic acid to treat patients. The duration of antibiotic therapy can be adjusted according to the patient’s condition. Most patients will experience clinical improvement within one week of starting treatment. If the patient is very ill at the time of presentation, parenteral therapy should be continued for 3 to 6 weeks and then switched to oral therapy when clinical symptoms improve. But it also depends on the location of the lesion. Generally primary cutaneous Nocardia can be treated for 1–3 months, lung and soft tissue infections should be treated for at least 6 months, disseminated Nocardia infections should be treated for 6–12 months, and cerebral nocardia should be treated for at least 12 months, the exact duration depending on response to treatment and resolution of the disease (Citation24,Citation25). In this case, the patient was hospitalized for 13 days and the lesion site was significantly reduced. After discharge, the patient continued to take amoxicillin-clavulanic acid orally for 8 days. Finally, the ulcer site crusted and fell off, and the patient has not recurred so far according to the follow-up.
4. Conclusion
Nocardia is often treated solely with TMP-SMX or TMP-SMX combined with antibiotics such as linezolid, imipenem and ceftriaxone. However, in recent years, Nocardia has increased resistance to these drugs, and sulfonamides, linezolid and other drugs have greater toxic and side effects on the host, so non-sulfonamides treatment deserves attention. The case of successfully treated of nocardiosis with amoxicillin-clavulanic acid provides a reference scheme for the treatment of sulfonamide-resistant Nocardia or patients who are intolerant to sulfonamides. And clinical treatment of Nocardia infection should pay more attention to strain identification and drug sensitivity results, rational drug use.
Ethics approval and consent to participate
This study was supported by the Ethics Committee of Zhejiang People’s Hospital (Ethics Committee Approval of Biomedical Research Involving Humans, Approval No.: 2022JS008) and was carried out in accordance with the ethical standards of the Declaration of Helsinki.
Consent for publication
Written and informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgements
We thank all members of the microbiology laboratory of Zhejiang Provincial People’s Hospital for their help in the collection of clinical data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the corresponding author.
Table 1. Antimicrobial susceptibility of the Nocardia brasiliensis by Kirby–Bauer method and Epsilometer test.
Additional information
Funding
References
- Margalit I, Lebeaux D, Tishler O, et al. How do I manage nocardiosis? Clin Microbiol Infect. 2021;27(4):1–4. doi:10.1016/j.cmi.2020.12.019.
- Lv H, Chen M, Ji Y, et al. A rare case of a subcutaneous abscess caused by Nocardia cyriacigeorgica in an immunocompetent patient. Infect Drug Resist. 2023;16:263–268. doi:10.2147/IDR.S395333.
- Toyokawa M, Ohana N, Ueda A, et al. Identification and antimicrobial susceptibility profiles of nocardia species clinically isolated in Japan. Sci Rep. 2021;11(1):16742. doi:10.1038/s41598-021-95870-2.
- Yi M, Wang L, Xu W, et al. Species distribution and antibiotic susceptibility of Nocardia isolates from Yantai, China. Infect Drug Resist. 2019;12:3653–3661. doi:10.2147/IDR.S232098.
- Berges Gimeno MP, Bernal Rubio L, Roldán E, et al. Positive basophil activation test result in a patient with anaphylaxis to cotrimoxazole. J Investig Allergol Clin Immunol. 2022;32(5):410–412. doi:10.18176/jiaci.0778.
- Perazella MA. Crystal-induced acute renal failure. Am J Med. 1999;106(4):459–465. doi:10.1016/s0002-9343(99)00041-8.
- Jahncke A, Kay V, Fiore B. Tattoo trouble: a case of drug-induced thrombocytopenic purpura. Mil Med. 2022;187(5–6):e778–e780. doi:10.1093/milmed/usab068.
- Lin J, Ding J, Di X, et al. Association between prenatal antibiotics exposure and measures of fetal growth: a repeated-measure study. Ecotoxicol Environ Saf. 2022;244:114041. doi:10.1016/j.ecoenv.2022.114041.
- Hamdi AM, Fida M, Deml SM, et al. Retrospective analysis of antimicrobial susceptibility profiles of nocardia species from a tertiary hospital and reference laboratory, 2011 to 2017. Antimicrob Agents Chemother. 2020;64(3):e01868-19. doi:10.1128/AAC.01868-19.
- Engelbrecht A, Saad H, Gross H, et al. Natural products from nocardia and their role in pathogenicity. Microb Physiol. 2021;31(3):217–232. doi:10.1159/000516864.
- Lebeaux D, Freund R, van Delden C, et al. Outcome and treatment of nocardiosis after solid organ transplantation: new insights from a European study. Clin Infect Dis. 2017;64(10):1396–1405. doi:10.1093/cid/cix124.
- Fujita T, Ikari J, Watanabe A, et al. Clinical characteristics of pulmonary nocardiosis in immunocompetent patients. J Infect Chemother. 2016;22(11):738–743. doi:10.1016/j.jiac.2016.08.004.
- JW,Wilson. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi:10.1016/j.mayocp.2011.11.016.
- Li S, Ji B, Teng Y, et al. Erythema nodosum following nocardia infection: a case report. Medicina. 2022;58(12):1873. doi:10.3390/medicina58121873.
- Camozzota C, Goldman A, Tchernev G, et al. A primary cutaneous nocardiosis of the hand. Open Access Maced J Med Sci. 2017;5(4):470–472. doi:10.3889/oamjms.2017.106.
- Ramos-E-Silva M, Lopes RS, Trope BM. Cutaneous nocardiosis: a great imitator. Clin Dermatol. 2020;38(2):152–159. doi:10.1016/j.clindermatol.2019.10.009.
- Secchin P, Trope BM, Fernandes LA, et al. Cutaneous nocardiosis simulating cutaneous lymphatic sporotrichosis. Case Rep Dermatol. 2017;9(2):119–129. doi:10.1159/000471788.
- Lao CK, Tseng MC, Chiu CH, et al. Clinical manifestations and antimicrobial susceptibility of nocardia species at a tertiary hospital in Taiwan, 2011–2020. J Formos Med Assoc. 2022;121(10):2109–2122. doi:10.1016/j.jfma.2022.06.011.
- Barry M, AlShehri S, Alguhani A, et al. A fatal case of disseminated nocardiosis due to Nocardia otitidiscaviarum resistant to trimethoprim-sulfamethoxazole: case report and literature review. Ann Clin Microbiol Antimicrob. 2022;21(1):17. doi:10.1186/s12941-022-00511-9.
- Tan YE, Chen SC, Halliday CL. Antimicrobial susceptibility profiles and species distribution of medically relevant nocardia species: results from a large tertiary laboratory in Australia. J Glob Antimicrob Resist. 2020;20:110–117. doi:10.1016/j.jgar.2019.06.018.
- Davidson N, Grigg MJ, Mcguinness SL, et al. Safety and outcomes of linezolid use for nocardiosis. Open Forum Infect Dis. 2020;7(4):ofaa090.
- Sim BZ, Aaron L, Coulter C, et al. A multi-Centre retrospective study of nocardia speciation and antimicrobial susceptibility in Queensland, Australia. Eur J Clin Microbiol Infect Dis. 2023;42(3):339–345. doi:10.1007/s10096-022-04542-0.
- Yang J, Ren HT, Wang J, et al. Clinical characteristics, susceptibility profiles, and treatment of nocardiosis: a multicenter retrospective study in 2015-2021. Int J Infect Dis. 2023;130:136–143. doi:10.1016/j.ijid.2023.02.023.
- Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38(2):89–97. doi:10.1007/s15010-009-9193-9.
- Restrepo A, Clark NM. Nocardia infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American society of transplantation. Clin Transplant. 2019;33(9):e13509. doi:10.1111/ctr.13509.