Dear Editor,
Acrodermatitis continua of Hallopeau (ACH) is a rare variant of localized pustular psoriasis characterized by chronic recurrent eruptions of sterile pustules of the distal fingers and sometimes toes, as well as the nail apparatus (Citation1). Pustules of the nail bed and matrix can lead to complications, such as onychodystrophy, progressive nail apparatus destruction, osteolysis, and arthritic joint involvement (Citation2,Citation3). Due to ACH’s chronic and relapsing nature, long-term control is needed to prevent these adverse outcomes. Pathogenesis is not fully understood, but in some patients, mutations in the IL36RN gene have been found (Citation4). The diagnosis is based on clinical and histopathological examination. Gram stain, culture and potassium hydroxide preparation should be performed to rule out bacterial infection, candidiasis and dermatophytosis (Citation5). Due to the rarity of this condition, no standardized guidelines exist (Citation6). Patients who fail to improve following traditional systemic conventional therapies, such as acitretin, methotrexate or cyclosporine, are contraindicated or fail; monoclonal antibodies can be used as a valid alternative. However, data on their effectiveness for the treatment of pustular psoriasis are limited (Citation6). We report a case of an ACH successfully treated with risankizumab, a humanized IgG1 monoclonal antibody that selectively binds to the interleukin-23 (IL-23) cytokine and blocks its interaction with the IL-23 receptor (Citation7).
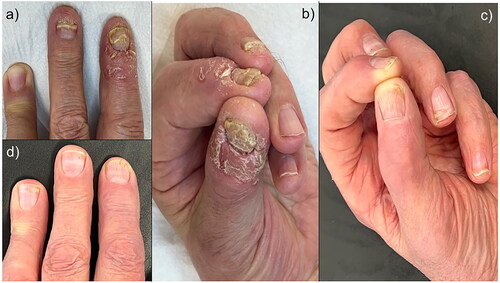
A 79-year-old male patient was referred to our department for a 2-year history of recurrent, painful, redness, swelling, pustules and tenderness on the tips of several digits on the left hand. Clinical examination showed edema, erythema, crusts and coalescent pustules of the nail fold and fingertip, with concomitant onychodystrophy (). The patient reported a pain score of 9 on the visual analog scale (VAS) and 20 points on the Dermatology Life Quality Index (DLQI). According to the clinical course of the disease and repeated clinical appearance of the manifestations, the patient was diagnosed with ACH. His past medical history was unremarkable, blood tests were within normal range, and he was not on any medications. He was later treated with acitretin (25 mg/day for 4 months), topical calcipotriene, and betamethasone dipropionate foam without any clinical benefits. Because of the patient’s age, we decided to use risankizumab owing to its good safety profile as an anti-IL-23 drug. Risankizumab was administered according to the dose for plaque psoriasis, 150 mg subcutaneously at weeks 0 and 4, and then every 12 weeks. On follow-up after 16 weeks of regular control, almost complete resolution of the lesions, including nails, was observed (). We observed progressive negativization of the VAS pain scale and DLQI) with scores of 0 points.
Figure 1. (a,b): Oedema, erythema, crusts and coalescent pustules of the nail fold and fingertip, with concomitant onychodystrophy. (c,d): Complete resolution of the lesions, including nails.
Due to its low prevalence, no treatment guidelines or randomized controlled clinical trials exist, so the treatment for ACH is extremely challenging.
Biological therapies play a key role in treating psoriasis and are a valid option also for pustular variants, including ACH (Citation8,Citation9). The therapeutic approach must be individualized according to the patient’s clinical characteristics and comorbidities.
Several authors have shared the experience of using biological agents for ACH based on case reports and small case series, with the anti-TNF agents being the most commonly reported.
However, anti-TNF agent therapy often loses efficacy over time, prompting a switch to other agents (Citation10,Citation11) or requiring an increased dose and frequency to maintain efficacy (Citation12,Citation13).
In addition, cases of ACH treated with anti-IL-17 monoclonal antibodies with different results have also been reported (Citation14–16). Furthermore, risankizumab, after two initiation doses at Week 0 and Week 4, requires only four administrations/year versus one administration every 2 weeks with anti-TNF treatment. Given the age of the patients and the similar efficacy and tolerability profile of risankizumab and anti-TNF agents, we have preferred a simpler and more convenient administration schedule.
We have identified two case reports of patients with ACH treated with risankizumab (Citation17,Citation18). However, to our knowledge, the case we described is the first case of an elderly patient with ACH treated with risankizumab. Furthermore, the response was more rapid and evident than in the other documented cases. Indeed, in our patient, risankizumab allowed an exceptionally rapid remission; 4 weeks after the first dose, a considerable alleviation of pain and psoriatic lesions were observed. At 16 weeks, the skin and nail lesions were almost completely cleared in all affected fingers, resulting in a consistently improved quality of life due to the disease-related impairment of a critical functional localization, such as the hands.
To our knowledge, this is the only documented case in which risankizumab guaranteed a rapid and complete resolution of both skin and nail manifestations.
Further studies are needed to establish the exact role of risankizumab in managing ACH; however, our case report suggests that risankizumab might be considered a possible valid choice for managing ACH.
Acknowledgments
Editorial assistance was provided by Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Data availability statement
Additional data supporting the findings of this manuscript are available upon reasonable request to the corresponding author.
Additional information
Funding
References
- Smith MP, Ly K, Thibodeaux Q, et al. Acrodermatitis continua of Hallopeau: clinical perspectives. Psoriasis (Auckl). 2019;9:1–3. doi: 10.2147/PTT.S180608.
- Sehgal VN, Verma P, Sharma S, et al. Acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50(10):1195–1211. doi: 10.1111/j.1365-4632.2011.04993.x.
- Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25(6):510–518. doi: 10.1016/j.clindermatol.2007.08.003.
- Chularojanamontri L, Rattanakorn K, Julanon N, et al. Acrodermatitis continua of Hallopeau and generalised pustular psoriasis: should they be the same or different entities? Exp Dermatol. 2023. Epub ahead of print. doi: 10.1111/exd.14805.
- Sehgal VN, Sharma S. Significance of Gram’s stain smear, potassium hydroxide mount, culture, and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9(4):260–261.
- Menter A, Van Voorhees AS, Hsu S. Pustular psoriasis: a narrative review of recent developments in pathophysiology and therapeutic options. Dermatol Ther (Heidelb). 2021;11(6):1917–1929. doi: 10.1007/s13555-021-00612-x.
- Orsini D, Gargiulo L, Ibba L, et al. Effectiveness of risankizumab in plaque psoriasis with involvement of difficult-to-treat areas: a real-world experience from two referral centers. J Dermatolog Treat. 2023;34(1):2220849. doi: 10.1080/09546634.2023.2220849.
- Gargiulo L, Toso F, Ibba L, et al. Biologics for the treatment of severe acrodermatitis continua of hallopeau: report of two cases successfully treated with ixekizumab and ustekinumab. Dermatol Pract Concept. 2023;13(2):e2023103. doi: 10.5826/dpc.1302a103.
- Narcisi A, Bernardini N, Orsini D, et al. Long-term safety and efficacy of adalimumab in psoriasis: a multicentric study focused on infections (connecting study). Postepy Dermatol Alergol. 2020;37(3):428–434. doi: 10.5114/ada.2020.96910.
- Palacios-Álvarez I, Simal-Gómez G, Mas-Vidal A, et al. Treatment of acrodermatitis continua of Hallopeau with ustekinumab as monotherapy after failure of anti-TNF agents. J Dtsch Dermatol Ges. 2018;16(5):611–613. doi: 10.1111/ddg.13506.
- Gokdemir G, Kutlu S, Köşlü A. Acrodermatitis continua resistant to etanercept: therapeutic challenge and unfortunate outcome. J Eur Acad Dermatol Venereol. 2009;23(3):345–347. doi: 10.1111/j.1468-3083.2008.02849.x.
- Tobin AM, Kirby B. Successful treatment of recalcitrant acrodermatitis continua of Hallopeau with adalimumab and acitretin. Br J Dermatol. 2005;153(2):445–446. doi: 10.1111/j.1365-2133.2005.06759.x.
- Ryan C, Collins P, Kirby B, et al. Treatment of acrodermatitis continua of Hallopeau with adalimumab. Br J Dermatol. 2009;160(1):203–205. doi: 10.1111/j.1365-2133.2008.08893.x.
- Passante M, Dastoli S, Nisticò SP, et al. Effectiveness of brodalumab in acrodermatitis continua of Hallopeau: a case report. Dermatol Ther. 2020;33(1):e13170.
- Miller AC, Holland TE, Cohen DJ. Treatment of acrodermatitis continua of hallopeau with ixekizumab. J Dermatolog Treat. 2021;32(1):117–119. doi: 10.1080/09546634.2019.1628170.
- Schmid E, Mohr J, Schön MP, et al. Two cases of acrodermatitis continua suppurativa (Hallopeau’s disease) treated with IL-17A inhibitors. J Dtsch Dermatol Ges. 2019;17(6):643–645. doi: 10.1111/ddg.13857.
- Langer N, Wilsmann-Theis D, Kromer C, et al. Successful therapy of acrodermatitis continua of Hallopeau with IL-23 blockers -two new cases. J Dtsch Dermatol Ges. 2021;19(10):1504–1507. doi: 10.1111/ddg.14586.
- Hugo J, Gkalpakioti P, Arenbergerova M, et al. Acrodermatitis continua Hallopeau successfully treated by risankizumab. Int J Dermatol. 2021;60(4):e144–e145. doi: 10.1111/ijd.15391.

