Abstract
Objectives
The purpose of this study was to analyze the drug survival rate of dupilumab up to 2 years in a large real-world cohort of adult patients affected by moderate/severe atopic dermatitis (AD), and to investigate the clinical, demographic and predictive factors influencing the patients’ treatment persistence.
Material and methods
This study included adult patients affected by moderate-to-severe AD treated with dupilumab for at least 16 weeks who visited 7 dermatologic outpatient clinics in Lazio, Italy, from January 2019 until August 2021.
Results
A total of 659 adult patients (345 male [52.3%], mean age: 42.8 years) with an average treatment duration of 23.3 months were enrolled in the study. Overall, 88.6% and 76.1% of patients were still on treatment after 12 and 24 months, respectively. The drug survival rate for discontinuation due to AEs and dupilumab ineffectiveness was 95.0% at 12 months and 90.0% at 24 months. The main reasons for drug discontinuation included inefficacy (29.6%), failed compliance (17.4%), persistent efficacy (20.4%) and adverse events (7.8%). Adult AD onset (≥18 years) and EASI score severity measured at the last follow-up visit were the only factors significantly associated with lower drug survival.
Conclusion
This study revealed an increased cumulative probability of dupilumab survival at 2 years, reflected by a sustained effectiveness and a favorable safety profile of the drug.
Keywords:
Introduction
Atopic dermatitis (AD) is the most common inflammatory skin disease, with a prevalence of 20% in children and 5–10% in adults, and over 230 million people are affected worldwide (Citation1). It is characterized by intense itch, dry skin and eczematous lesions showing preferential involvement of body areas according to the patient’s age (Citation1). Severe AD is often associated with poor sleep quality, mental health disturbances, including anxiety and depression, and overall impairment of the patient’s quality of life (Citation1–3). Over one-third of patients suffer from a moderate/severe form of AD, which requires systemic treatment to be adequately controlled (Citation4). Traditional systemic immunosuppressant’s commonly used in AD, including systemic corticosteroids, cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have not been investigated by randomized studies and are associated with systemic toxicity and poor tolerability (Citation4). Dupilumab is a monoclonal antibody targeting the IL-4 alpha chain and thus inhibiting both IL-4 and IL-13 signaling. Since its release in Europe in September 2017, dupilumab has profoundly changed the management of moderate/severe AD in adults, allowing long-term control of signs and symptoms of the disease and a favorable safety profile (Citation5–7). The most recent data of the open-label study ‘LIBERTY AD OLE’ reported a sustained improvement in all clinical efficacy outcomes up to 4 years of treatment, with a safety profile comparable to that described in previous controlled trials of up to 52 weeks (Citation8). In addition, several real-life experiences have confirmed a remarkable long-term activity and safety profile of dupilumab, with low rates of drug discontinuation consistent with those shown in randomized clinical trials (2.2%-11.5%) (Citation5–7,Citation9–11).
Drug survival is a comprehensive outcome defined as the length of time an individual takes a drug until it is discontinued. In cases of chronic skin diseases, drug survival incorporates patient satisfaction, efficacy, safety, and tolerability of treatments in real life (Citation12).
The aim of our study was to analyze the drug survival rate of dupilumab up to 2 years in a large real-world population of adult patients with moderate/severe AD and to investigate the clinical, demographic, and predictive factors influencing the patients’ treatment persistence.
Patients and methods
In this retrospective multicenter study, we included adult patients with moderate to severe AD under treatment with dupilumab for at least 16 weeks who were referred to 7 dermatologic outpatient clinics in Lazio, Italy, from January 2019 until August 2021. Dupilumab was prescribed according to the Italian Drug Agency (AIFA) recommendations (Eczema Area Severity Index (EASI) score ≥24, inadequate response to or intolerance to Cyclosporin A (CsA) or medical condition not compatible with CsA treatment).
All patients enrolled in this study used daily moisturizers, while topical medium- to very-high-potency corticosteroids and topical calcineurin inhibitors were applied as needed.
The following demographic and clinical data were collected from each patient: region of residence, age, sex, AD features (age at AD onset, clinical phenotypes according to Silvestre Salvador et al. classification (Citation13), topographical distribution of skin lesions, AD severity, previous treatments) and dupilumab therapy (time duration, discontinuation, reasons for discontinuation and adverse events). Clinical outcomes were collected at the following timepoints: baseline, week 16, week 48, week 64, week 80 and week 96. Disease severity was assessed by (a) Eczema Area Severity Index (EASI) score, which varies from 0 to 72; (b) itch Numeric Rating Scale [itch-NRS] ranging from 0 to 10]; and (c) Dermatology Life Quality Index (DLQI) with a scale from 0 to 30.
Drug survival was considered the period between the start of drug intake and drug discontinuation. In line with previous studies, treatment discontinuation was defined as dupilumab interruption for more than 2 months (Citation11). Reasons for drug discontinuation were categorized as adverse events (AEs), sustained remission of disease, loss of efficacy, poor adherence, and other reasons, including pregnancy, concomitant diseases, and death from COVID-19 infection and vaccination.
The study was approved by the Local Ethical Committee (protocol n. 0054579/2022).
Statistical analysis
Continuous variables were described as the means and standard deviations (SD), and categorical variables were reported using absolute and relative (%) frequencies. Kaplan–Meier survival plots were obtained to define descriptive unadjusted DS curves. Overall discontinuation and discontinuation due to dupilumab ineffectiveness and AEs were analyzed separately as failure events for drug survivor function. Patients were censored if the reason for discontinuation did not match the event of interest or if lost to follow-up. The percentage of patients still on treatment at 12 and 24 months after the start of dupilumab was used to calculate the cumulative survival rates. To estimate adjusted hazard ratios (HRs) for therapy discontinuation, multivariable Cox regression models were calculated, including sex, age at disease onset, age at treatment initiation, and previous cyclosporin intake as clinically meaningful variables that could be relevant for the model. We considered including the predictor variable in the final model if the univariate Cox proportional hazard regression test for continuous variables or the log-rank test of equality for categorical variables had a p value of 0.2–0.25 or less. Analyses were performed by using STATA/BE 17.0 Software (StataCorp, Texas).
Results
Clinical and demographic characteristics of the general population
Overall, 659 adult subjects (345 male [52.3%] 312 females [47.7%], mean age: 42.8 ± 19.90) were included in the study. Demographic and clinical data are summarized in .
Table 1. Clinical and demographic characteristics of the study population.
The majority of dupilumab-treated patients [481/570 (87.1%)] referred to the prescribing centers for drug supply in Lazio, while 71/570 (12.9%) patients were residents outside the Lazio region. Most patients reported the onset of disease during childhood (0–11) (49.8%, 328/659) and adulthood (18–64) (32.8%, 216/659), while the occurrence of AD during adolescence (Citation11–18) and old age (>64) was less frequently observed ().
The classic clinical phenotypes (lichenified/exudative flexural dermatitis, mostly associated with head-and-neck eczema and/or hand eczema) were diagnosed in 49.3% (177/359) of the study population, followed by the generalized pattern (36.5%, 133/359), prurigo nodularis (7.2%, 26/359) and nummular eczema (5.3%, 19/359). Concerning sensitive body areas, 12/122 patients (9.8%) showed involvement of the head and neck area, while 17/122 patients (13.9%) had hand involvement.
Previous systemic treatment with cyclosporine A was reported in 55.6% (366/659) of patients, while in the remaining study population (44.4%, 292/659), cyclosporine was not previously prescribed because of medical contraindications (e.g., hypertension, nephropathy).
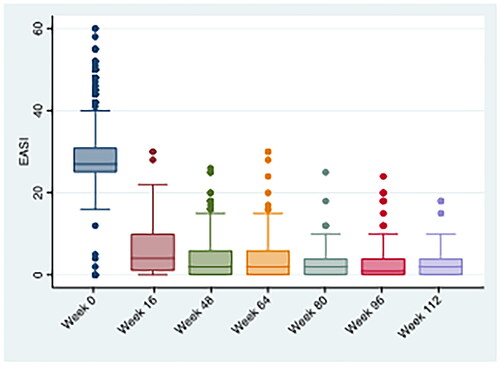
At the time of dupilumab initiation, the mean (±SD) EASI score was 28.0 (±6.7), the mean (±SD) itch-NRS was 8.2 (±1.9), and the mean (±SD) DLQI was 15.9 (±7.5).
Drug survival
Among 659 patients, 580 (88.0%) had available start and end data of dupilumab treatment (or last follow-up date) to use for time to event analysis. A total of 115/580 (19.8%) patients discontinued dupilumab therapy. The main reasons for discontinuation were inefficacy in 29.6% (34/115), failed compliance in 17.4% (20/115), stable efficacy in 20.8% (23/115), and adverse events in 7.8% (9/115). Concerning the side effects that led to drug discontinuation, 5/9 (55.6%) cases were ocular side effects (xerosis, conjunctivitis), and 4/9 (44.4%) were injection site adverse reactions.
Dupilumab discontinuation was significantly more frequent in adult AD patients (≥18 years) (24.2%, 55/226) than in those with early (<18 years) onset (15.1%, 45/297) (p = .008). No other association with demographic features was observed.
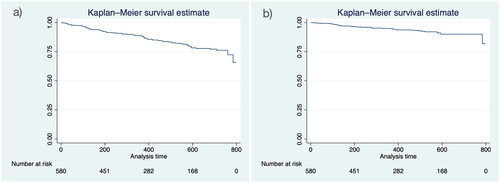
The average (±SD) treatment duration was 23.3 months (±16.8). Considering overall discontinuation as a failure event for drug survival, 88.6% (95% CI: 85.5%–91.1%) and 76.1% (95% CI: 70.8%–80.7%) of patients remained on dupilumab at 12 and 24 months, respectively (). The cumulative probability of drug survival considering discontinuation due to either AEs or ineffectiveness was 95.0% (95% CI: 92.6%–96.6%) at 12 months and 90.0% (95% CI: 86.1%–92.9%) at 24 months ().
Figure 1. Kaplan–meier survival plots. (a) Overall discontinuation and (b) discontinuation due to AEs and inefficacy were considered failure events for drug survivor function (analysis time = days).
The univariate analysis showed that adult AD onset (≥18 years) (HR = 1.592, 95% CI = 1.052–2.409, p = .028) and EASI score at the last follow-up visit (HR = 1.128, 95% CI = 1.099–1.158, p < .0001) were associated with an increased risk of overall discontinuation, while for each unit increase in the EASI score assessed at the last follow-up visit, a 16% additional risk of discontinuation due to ineffectiveness or AE was observed. Disease severity assessed by absolute EASI score at the last follow-up visit was the only predictive factor for overall drug discontinuation, as confirmed by multivariate analysis, with a 13% increased likelihood of therapy discontinuation for each unit increase in the EASI score (HR = 1.13, 95% CI = 1.104–1.166, p < .0001), regardless of baseline EASI score, age at disease onset, sex, age at treatment initiation and previous cyclosporin administration (Prob > chi2 < .0001). The same trend was observed in the case of drug discontinuation for ineffectiveness and AEs (HR = 1.17, 95% CI = 1.123–1.223, p < .0001) ().
Table 2. Dupilumab efficacy at specified time points considering the overall study population.
Table 3. Univariable and multivariable Cox proportional hazard regression model predicting overall drug discontinuation (Prob > chi2 = 0.0000) and discontinuation due to AEs and inefficacy (Prob > chi2 = 0.0000).
Effectiveness of dupilumab throughout 2 years of observation
Dupilumab was effective in the long-term management of AD as demonstrated by the progressive decrease in the mean (±SD) EASI score, which dropped from 29.2 (±7.6) to 2.9 (±4.5; p < .0001) throughout the 2-year observation period (). The amelioration of clinical manifestations was accompanied by a marked reduction in itch ([mean (±SD) itch-NRS from 8.3 (± 1.9) to 1.7 (±2.2), p < .0001] and progressive recovery of the overall quality of life [mean (±SD) DLQI from 15.9 (±7.5) to 2.1 (±4.6), p < .0001] from baseline to the last follow-up ().
Discussion
Long-term control of moderate-to-severe AD has been extremely challenging with a therapeutic armamentarium limited to conventional immunosuppressant therapies (Citation4). Currently, dupilumab has completely revolutionized the management of adult AD, permitting the achievement of long-term remission of disease signs and symptoms in an increased proportion of patients (Citation6).
The present study confirms an optimal long-term safety profile and effectiveness of dupilumab in 659 adult subjects with moderate-to-severe AD, reflecting a high drug survival rate, up to 2 years of observation. In particular, the overall drug survival rates were 88.6% and 76.1% at 1 and 2 years, respectively. These results are consistent with data recently published from a real-world Canadian multicenter study on 145 patients, which showed a two-year overall drug survival rate of 80% (Citation14), whereas other real-world experiences reported slightly lower rates compared to our study (Citation10,Citation15,Citation16). Considering drug discontinuation exclusively due to AEs and/or ineffectiveness, the cumulative probability of drug survival at 1 and 2 years was 95.0% and 90.0%, respectively.
In line with our findings, a real-world experience comparing drug survival rates of dupilumab, methotrexate and cyclosporine reported dupilumab discontinuation after one year of treatment due to poor response and/or AEs in 2% of treated patients, whereas approximately half of patients treated with methotrexate or cyclosporine interrupted treatment (Citation16).
Long-term effectiveness in controlling AD manifestation, limited occurrence of AEs, the absence of drug interaction, and organ toxicity were the main reasons explaining the significantly higher drug survival rates reported with dupilumab in comparison with traditional immunosuppressants (Citation16). In our study, 115/580 (19.8%) patients withdrew from dupilumab treatment, most frequently due to ineffectiveness, which was reported in 34/580 (5.9%) patients.
Khosravi et al. (Citation10), in a 2-year drug survival analysis including 112 AD patients treated with dupilumab, reported similar findings, describing disease flares as the main reason for drug discontinuation (5%), followed by conjunctivitis (3%), and satisfactory control of AD combined with phototherapy in 0.9%. Notably, the authors did not detect any association between demographic characteristics and drug failure (Citation9), while in our study, drug discontinuation was more frequently associated with adult AD onset (24.2%) than early onset (15.1%) (p = .008). In addition, adult AD onset was found to increase the risk of overall discontinuation by 59% (HR = 1.59, p = .028) in the univariable Cox regression analysis, although this finding was not confirmed in the multivariable model. Notably, Dal Bello et al. in a 16-month drug survival analysis on 149 patients, found that an increased risk of drug discontinuation was associated with adult AD onset and shorter disease duration (Citation17). The authors hypothesized that the higher persistence of treatment in patients with an earlier AD onset may be partially explained by the central role of Th2 inflammation found in these patients compared to the mixed Th2/Th17/Th22 skewing phenotype detected in patients with adult AD onset (Citation17).
In contrast to these observations, a recent multicentric study, including 363 AD patients under treatment with dupilumab throughout 4 years of observation, identified the early onset of AD (before the age of 18 years) as a significant predictor of shorter overall drug survival (Citation18).
It must be emphasized that the combination of a traditional systemic immunosuppressant or phototherapy with dupilumab may represent an effective strategy to enhance drug retention in patients experiencing an inadequate response to dupilumab monotherapy (Citation19,Citation20).
Indeed, considering the progressive consolidation of clinical results over time in up to 2 years of observation of dupilumab real-world experiences, the additional benefits obtained with combination therapy can then be maintained with dupilumab monotherapy (Citation21,Citation22).
The suspension of dupilumab due to satisfactory response according to the patient’s decision, regardless of whether shared with the dermatologist, is not uncommon (24/115, 20.9% of patients in our study), and it may negatively affect the cumulative probability of drug survival.
In our study, all patients who interrupted dupilumab for satisfactory response experienced complete clinical remission (EASI < 1; Itch-NRS < 1; DLQI < 1) for at least 6 months. Along these lines, a satisfactory and stable response to dupilumab was reported as one of the most frequent causes of drug interruption (Citation17,Citation23). The third reason for drug discontinuation was poor compliance with therapy (20/580, 3.4%), although the distance from the referral center was not the eventual reason explaining the poor compliance, as no significant differences in drug withdrawal were reported between patients residing in Lazio and extraregional residents.
Only 9/659 (1.4%) patients withdrew from dupilumab treatment because of drug-related AEs throughout the observation period of 122 weeks, thus confirming an excellent long-term safety profile. Drug interruption due to severe allergic conjunctivitis, recalcitrant to topical treatment with corticosteroid and cyclosporin, was observed in 5/659 (0.76%) subjects. This observation is consistent with other long-term real-world experiences reporting a discontinuation rate of dupilumab for ocular adverse events of lower than 2% (Citation10,Citation17,Citation24). Interestingly, C. Patruno et al. recently showed an amelioration of severe and recalcitrant dupilumab-induced conjunctivitis with an interval prolongation beyond the standard 2 weeks between drug administration (Citation25).
Another real-world multicenter study involving 715 adult AD patients treated with dupilumab estimated an overall drug survival for dupilumab of 90.3%, 85.9% and 78.6% at 1, 2 and 3 years, respectively (Citation15). Furthermore, when discontinuation for ineffectiveness was considered, drug survival increased significantly to 96.5%, 95.7% and 95.7% at the same timepoints, respectively. Similarly, when discontinuation for AE was considered, the cumulative probability of drug survival was 96.3%, 93.2% and 92.6% at 1, 2 and 3 years, respectively (Citation15). The use of immunosuppressive drugs at baseline and a lack of response at 4 weeks were identified as predictors of poor drug survival due to drug ineffectiveness, whereas use of immunosuppressive drugs at baseline, advanced age and very severe IGA resulted in shorter survival due to AEs.
In our study, a predictive value for dupilumab discontinuation was not observed for several clinical and demographic characteristics, including age, sex, baseline AD severity, clinical phenotype, involvement of special areas, and previous treatment with cyclosporine. The only predictive factor for dupilumab discontinuation was disease severity, defined as the absolute EASI score at the last follow-up visit, with a 14% increased likelihood of therapy discontinuation for each unit increase in the EASI score. This result implies that worsening AD is associated with an increased risk of discontinuation, with valuable clinical significance.
In this regard, it is important to emphasize the clinical relevance of identifying and avoiding, whenever possible, potential disease exacerbating factors (e.g., allergens, irritants) to optimize the persistence of patients under treatment.
The limitations of this study mainly consist of its retrospective nature. As drug survival is a comprehensive tool also influenced by therapeutic choices, the recent introduction of new drugs for the treatment of moderate to severe AD, including oral JAK inhibitors and tralokinumab, could affect the replicability of the results of this study in the future.
The coverage of drug costs in Italy is supported by the state as well as in many other Western countries, while in underdeveloped countries, financial reasons may limit the availability of dupilumab, and this must be considered a limitation that reduces the generalizability of the study’s results and conclusions.
In conclusion, the introduction of dupilumab filled an unmet therapeutic need in AD by providing prolonged effectiveness and tolerability that could not be achieved with previous conventional therapies. However, further research on the role of age and disease chronicity as well as on other potential predictive factors for treatment discontinuation is required to optimize and tailor the therapeutic approach to AD.
Acknowledgments
We thank the following individuals for their contribution to the collection of patient data: Eleonora De Luca, Marina Talamonti, and Salvatore Zanframundo.
Disclosure statement
NG is a speaker or board member for Abbvie, Sanofi Genzyme, and Leo-pharma; KP has served on an advisory board, received honoraria for lectures and/or research grants for Abbvie, Almirall, Lilly, Galderma, Leo Pharma, Pierre Fabre, Novartis, Sanofi, Sun Pharma, Janssen. AC has served as an advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Bristol Myers Squibb, Leo Pharma, Lilly, Janssen, Novartis, Pfizer and Sanofi Genzyme. BL is a speaker or board member for AbbVie, Amgen, Novartis, Almirall, Sunpharma, Sanofi Genzyme, Biogen, Eli Lilly, Janssen, Leo Pharma. GP declares that he has received honoraria from AbbVie, Leo-pharma, Novartis, Pfizer, and Sanofi. AS, ET, MM, MG, GM, RC, ADA, MS, VM, CP, CF, LC, PA, LDN, EDD, SP, VP and CP have no conflicts of interest to disclose.
Data availability statement
Enquiries related to the data generated or analyzed during this study can be directed to the corresponding author.
Additional information
Funding
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z.
- Gori N, Chiricozzi A, Marsili F, et al. National information campaign revealed disease characteristic and burden in adult patients suffering from atopic dermatitis. J Clin Med. 2022;11(17):5204. doi:10.3390/jcm11175204.
- Talamonti M, Galluzzo M, Silvaggio D, et al. Quality of life and psychological impact in patients with atopic dermatitis. J Clin Med. 2021;10(6):1298. doi:10.3390/jcm10061298.
- Siegels D, Heratizadeh A, Abraham S, et al. European academy of allergy, clinical immunology atopic dermatitis guideline group. Systemic treatments in the management of atopic dermatitis: a systematic review and meta-analysis. Allergy. 2021;76(4):1053–7. doi:10.1111/all.14631.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. SOLO 1 and SOLO 2 investigators: two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi:10.1056/NEJMoa1610020.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1.
- de Bruin-Weller M, Thaçi D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin a or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br J Dermatol. 2018;178(5):1083–1101. doi:10.1111/bjd.16156.
- Lauvelt A, Wollenberg A, Eichenfield LF, et al. No increased risk of overall infection in adults with moderate-to-severe atopic dermatitis treated for up to 4 years with dupilumab. Adv Ther. 2023;40(1):367–380. doi:10.1007/s12325-022-02322-y.
- Fargnoli MC, Esposito M, Ferrucci S, et al. A 48-week update of a multicentre real-life experience of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J Dermatolog Treat. 2022;33(2):1146–1149. doi:10.1080/09546634.2020.1773379.
- Khosravi H, Zhang S, Anderson AM, et al. Dupilumab drug survival, treatment failures, and insurance approval at a tertiary care center in the United States. J Am Acad Dermatol. 2020;82(4):1023–1024. doi:10.1016/j.jaad.2019.12.034.
- Silverberg JI, Guttman-Yassky E, Gadkari A, et al. Real-world persistence with dupilumab among adults with atopic dermatitis. Ann Allergy Asthma Immunol. 2021;126(1):40–45. doi:10.1016/j.anai.2020.07.026.
- Van den Reek JMPA, Kievit W, Gniadecki R, et al. Drug survival studies in dermatology: principles, purposes, and pitfalls. J Invest Dermatol. 2015;135(7):1–5. doi:10.1038/jid.2015.171.
- Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27(2):78–88. doi:10.18176/jiaci.0138.
- Georgakopoulos JR, Felfeli T, Drucker AM, et al. Two-year efficacy, safety, and drug survival of dupilumab for atopic dermatitis: a real-world Canadian multicenter retrospective study. JAAD Int. 2021;4:67–69. doi:10.1016/j.jdin.2021.06.001.
- Spekhorst LS, De Graaf M, Zuithoff NPA, et al. Dupilumab drug survival and associated predictors in patients with moderate to severe atopic dermatitis: long-term results from the daily practice BioDay registry. JAMA Dermatol. 2022;158(9):1048. doi:10.1001/jamadermatol.2022.3014.
- Spekhorst LS, Ariëns LF, Schaft J, et al. Two-year drug survival of dupilumab in a large cohort of difficult-to-treat adult atopic dermatitis patients compared to cyclosporine a and methotrexate: results from the BioDay registry. Allergy. 2020;75(9):2376–2379. doi:10.1111/all.14324.
- Dal Bello G, Maurelli M, Schena D, et al. Drug survival of dupilumab compared to cyclosporin in moderate-to-severe atopic dermatitis patients. Dermatol Ther. 2020;33(6):e13979.
- Pezzolo E, Rossi M, Caroppo F, et al. Long-term drug survival of dupilumab and associated predictors in moderate-to-severe atopic dermatitis: a real-world prospective cohort study. Acad Dermatol Venereol. 2023;37(6):18889. doi:10.1111/jdv.18889.
- Gori N, Chiricozzi A, Malvaso D, et al. Successful combination of systemic agents for the treatment of atopic dermatitis resistant to dupilumab therapy. Dermatology. 2021;237(4):535–541. doi:10.1159/000512890.
- Narla S, Silverberg JI, Simpson EL. Response to the article by Narla et al “Management of inadequate response and adverse effects to dupilumab in atopic dermatitis”. J Am Acad Dermatol. 2022;86(3):628–636. doi:10.1016/j.jaad.2021.06.017.
- Kojanova M, Tanczosova M, Strosova D, et al. Dupilumab for the treatment of atopic dermatitis: real-world data from the Czech Republic BIOREP registry. J Dermatolog Treat. 2022;33(5):2578–2586. doi:10.1080/09546634.2022.2043545.
- Gori N, Chiricozzi A, Peris K. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J Am Acad Dermatol. 2023;88(1):e65–e66. doi:10.1016/j.jaad.2022.09.055.
- Napolitano M, Mariano M, Cristaudo A, et al. Drug survival analysis of dupilumab and cyclosporin in patients with atopic dermatitis: a multicenter study. J Dermatolog Treat. 2022;33(5):2670–2673. doi:10.1080/09546634.2022.2067818.
- Nettis E, Fabbrocini G, Ortoncelli M, et al. Long-term effectiveness of dupilumab up to 52 weeks in atopic dermatitis in 253 adult patients. Br J Dermatol. 2021;184(3):561–563. doi:10.1111/bjd.19577.
- Patruno C, Potestio L, Fabbrocini G, et al. Dupilumab dose spacing after initial successful treatment or adverse events in adult patients with atopic dermatitis: a retrospective analysis. Dermatol Ther. 2022;35(12):e15933. doi:10.1111/dth.15933.