Dear Editor,
Secukinumab, an IL-17A monoclonal antibody, is effective for refractory hidradenitis suppurativa (HS). Nevertheless, only a few studies evaluating the effectiveness of secukinumab for HS in real-life setting are available (Citation1). The rates of patients achieving clinical response were 41% and 67% in two real-life studies, without any serious adverse effects (Citation2,Citation3). However, various paradoxical reactions related to the use of secukinumab including HS onset have been reported (Citation4). We report a case of pyoderma gangrenosum associated with Behçet’s-like disease induced by secukinumab in a male suffering from HS.
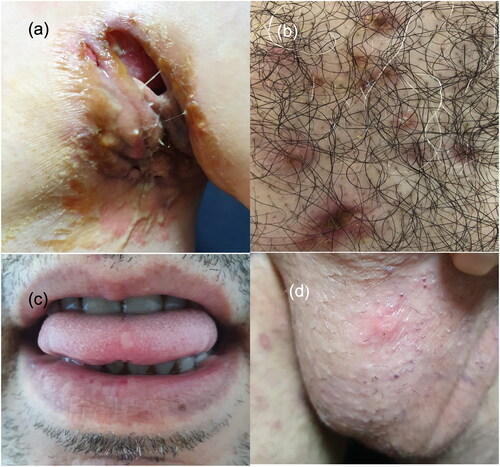
A 43-year-old diabetic man presented with a 14-years history of HS which was unresponsive to systemic antibiotics, isotretinoin, and adalimumab treatments. Due to Hurley stage III and refractory disease, adalimumab treatment was switched to secukinumab therapy. However, 10 days after the sixth dosage of secukinumab therapy, he noticed hyperemia and edema evolving to painful ulcerations on the axillary folds where diffuse sinus tracts and abscesses compatible with HS lesions were located (). Additionally, there were inflammatory pustules and papules on his sternum, back, legs, and buttocks (). Shortly after, some of these lesions on the legs and buttocks became ulcerated. During hospitalization, he developed a single genital ulcer and two oral ulcers that resolved spontaneously within a week. No recurrent or new ulcers developed thereafter ().
Figure 1. (a) Ulcerations with undermined and erythematous borders on the left axillary fold where diffuse sinus tracts and abscesses are also seen. (b) Hyperemic papulopustular lesions and an ulceration on the sternum. (c) and (d) Oral and genital aphthous ulcerations.
Punch biopsy of the axillary ulcer edge contained regenerative changes, neutrophil infiltration, and focal spongiosis. Fibroplasia, intense plasma cell infiltration, and foreign-body giant cells were present in all areas of the dermis. A second skin biopsy from the ulcer margin located on the left leg had small vessel vasculitis. The microbiologic culture from the ulceration site on the axillary fold was positive for Proteus mirabilis.
Pathergy test was negative, and blood analysis for HLA-B51 was positive. There was no ocular involvement according to the International Study Group criteria for Behçet’s Disease (BD). Although the histopathologic examination was compatible with both pyoderma gangrenosum and inflammatory flare of HS, the patient also had painful ulcers on the lower extremities and buttocks with violaceus border, undermined edges, and a history of pustules. In the clinical follow-up, these ulcers healed with wrinkled paper scars. Based on these findings, the final diagnosis of our patient was paradoxical ulcerative pyoderma gangrenosum and Behçet’s-like disease induced with secukinumab. Therefore, secukinumab was withdrawn.
The patient was treated with intravenous 4.5 mg piperacillin-tazobactam four times a day with good ulcer care, and colchicine (0.5 mg/three times a day) was started. Topical corticosteroid was applied on the papulopustular lesions and small sized ulcerations. After three weeks of therapy, the pain subsided and both granulation and re-epithelialization began.
HS is a chronic inflammatory disorder in which T helper (Th)1 and Th 17 cells and their inflammatory mediators including IL-17A and IL-17F contribute to the disease pathogenesis (Citation5). Currently, adalimumab is the only biologic approved for moderate to severe HS. However, the long-term effectiveness of adalimumab in daily practice has shown to be variable. Given the limited efficacy of the existing therapeutic agents, new therapies targeting the main inflammatory cytokines in HS are needed (Citation1).
Though secukinumab, an IL-17A monoclonal antibody, is effective in the treatment of HS, paradoxical HS associated with its usage has been reported (Citation4). Moreover, paradoxical pyoderma gangrenosum (PG) and exacerbation or triggering of Behçet’s-like disease following secukinumab have occurred () (Citation6–14). HLA-B51 was detected in 4 of 7 patients. Discontinuation of the suspected drug was the most common treatment.
Table 1. Patients’ characteristics with paradoxical pyoderma gangrenosum and Behçet’s like disease following secukinumab.
Our patient presented with paradoxical ulcerative PG and Behçet’s-like disease which belong to the spectrum of neutrophilic dermatoses. Neutrophilic dermatoses are a heterogeneous group of cutaneous inflammatory diseases characterized by the accumulation of neutrophils in the skin. Interleukin (IL)-17 is a driving force for the activation and migration of neutrophils (Citation15). After IL-17A inhibition by secukinumab, other forms of IL 17 as well as the pathogenic cytokines like IL-22 and IL-23 increase as compensatory mechanism. This leads to stimulation of neutrophil activation and paradoxical reaction. Impairment of the mucosal barrier due to inhibition of IL-17 and the triggering of innate inflammation by environmental microorganisms may also play a role in BD (Citation4). Bacteria may be involved in HS pathogenesis (Citation5). In our patient, IL-17 inhibition may have contributed to alteration of the cutaneous microbiome, leading to secondary bacterial infection (Proteus mirabilis) and subsequent disease progression with paradoxical reaction.
Although there are reports of similar paradoxical reactions separately in the literature, to our knowledge our patient is the first case who presented with Behçet’s-like disease and PG concurrently following secukinumab. Further studies and case reports to assess the safety of secukinumab would be required.
Acknowledgment
The patient in this manuscript has given written informed consent to publication of his case details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings are available from the corresponding author [CA] upon reasonable request.
Additional information
Funding
References
- Martora F, Megna M, Battista T, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:1–3. doi: 10.2147/CCID.S391356.
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181(3):609–611. doi: 10.1111/bjd.17822.
- Ribero S, Ramondetta A, Fabbrocini G, et al. Effectiveness of secukinumab in the treatment of moderate-severe hidradenitis suppurativa: results from an Italian multicentric retrospective study in a real-life setting. J Eur Acad Dermatol Venereol. 2021;35(7):e441–e442. doi: 10.1111/jdv.17178.
- Messina F, Piaserico S. The dark side of the moon: the immune-mediated adverse events of IL-17A/IL-17R inhibition. J Dermatolog Treat. 2022;33(5):2443–2454. doi: 10.1080/09546634.2022.2062281.
- Wolk K, Join-Lambert O, Sabat R. Aetiology and pathogenesis of hidradenitis suppurativa. Br J Dermatol. 2020;183(6):999–1010. doi: 10.1111/bjd.19556.
- Shiga H, Fukuda S, Iijima K. Interleukin-17A inhibitor-induced Crohn’s disease/Behçet’s disease-like lesions. Inflamm Bowel Dis. 2017;23(6):E38–E39. doi: 10.1097/MIB.0000000000001142.
- Grimaux X, Leducq S, Goupille P, et al. Ulcérations buccales aphtoïdes inaugurales d’une maladie inflammatoire chronique de l’intestin induite par le sécukinumab [Aphthous mouth ulcers as an initial manifestation of sécukinumab-induced inflammatory bowel disease]. Ann Dermatol Venereol. 2018;145(11):676–682. French. doi: 10.1016/j.annder.2018.07.009.
- Dincses E, Yurttas B, Esatoglu SN, et al. Secukinumab induced Behçet’s syndrome: a report of two cases. Oxf Med Case Reports. 2019;31(5):omz041.
- Barrado-Solís N, Rodrigo-Nicolás B, De la Morena-Barrio I, et al. Report of two cases of behçet’s disease developed during treatment with secukinumab. J Eur Acad Dermatol Venereol. 2020;34(10):e587–e589. doi: 10.1111/jdv.16454.
- Calleja Algarra A, Aragón Miguel R, Andrés Lencina JJ, et al. Behçet’s-like disease during secukinumab treatment: new paradoxical reaction? J Dtsch Dermatol Ges. 2021;19(1):116–118. doi: 10.1111/ddg.14196.
- Jin K, Matsuzaki Y, Akasaka E, et al. Pyoderma gangrenosum triggered by switching from adalimumab to secukinumab. J Dermatol. 2019;46(3):e108-9–e109. doi: 10.1111/1346-8138.14611.
- Wollina U, Schönlebe J, Fürll C. Pyoderma gangrenosum induced by secukinumab-A late paradoxical drug reaction. Dermatol Ther. 2020;33(1):e13161.
- Petty AJ, Whitley MJ, Balaban A, et al. Pyoderma gangrenosum induced by secukinumab in a patient with psoriasis successfully treated with ustekinumab. JAAD Case Rep. 2020;6(8):731–733. doi: 10.1016/j.jdcr.2020.06.011.
- Orita A, Hoshina D, Hirosaki K. Pyoderma gangrenosum caused by secukinumab successfully treated with risankizumab: a case report and literature review. Clin Exp Dermatol. 2022;47(7):1372–1374. doi: 10.1111/ced.15183.
- Weiss EH, Ko CJ, Leung TH, et al. Neutrophilic dermatoses: a clinical update. Curr Dermatol Rep. 2022;11(2):89–102. doi: 10.1007/s13671-022-00355-8.

