Abstract
Background
Dupilumab is a monoclonal antibody against the IL-4/IL-13 receptor-subunit approved for the treatment of moderate-severe atopic dermatitis (AD). Some attempts to increase dose interval have been described in both trial and real-world settings.
Objective
This study aimed to identify predictive clinical and demographic factors affecting patient selection for dose spacing or treatment withdrawal due to satisfactory response.
Materials and methods
This retrospective study included adult patients with moderate-to-severe AD treated with dupilumab for at least 16 weeks. Descriptive statistics were performed to analyze demographic and clinical variables. Logistic regression models were used to identify predictor variables.
Results
A total of 818 adult patients with moderate-to-severe AD was included in the study and 12% (97/818) of them performed dose spacing to 3–4 weeks or treatment withdrawal (8%, 67/818). The presence of non-cutaneous atopic manifestations (OR = 1.59, 95%CI = 1.06–2.38, p = 0.024), prurigo nodularis phenotype (OR = 4.5, 95%CI = 1.87–10.9, p = 0.001) and the age at treatment initiation (OR = 1.82, 95%CI = 1.12–2.94, p = 0.015) were confirmed as the strongest predictors of dose spacing or treatment withdrawal while maintaining dupilumab effectiveness.
Conclusion
Our findings contribute to define the patient profile that could maintain the therapeutic response after dose spacing or treatment withdrawal.
Key message
Predicting factors identified patients with dupilumab who could benefit of dose spacing or treatment withdrawal.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting 2.5% of the worldwide population (Citation1).
The standard of care encompasses topical agents and/or phototherapy for mild or localized forms while conventional systemic immunomodulants, phototherapy, and targeted therapies are used for the management of moderate-to-severe forms (Citation2, Citation3).
The first biologic agent approved for the treatment of moderate-severe AD was dupilumab, a monoclonal antibody against the IL-4 receptor α-subunit that inhibits both IL-4- and IL-13-mediated signaling (Citation4). Its efficacy and safety have been well established in clinical trials and real-world studies (Citation5–7). In adults, the approved dupilumab dosage consists of 600 mg as staring dose, followed by 300 mg every 2 weeks. Attempts to increase dose interval have been described in both trial and real-world settings (Citation8–12). Most of the reports showed that clinical remission, obtained with approved dosages, was maintained in a subset of patients after interval prolongation beyond the standard 2-weeks between dupilumab administrations (referred as dose spacing) (Citation8–10). These positive effects were meant as a sustained response to dupilumab treatment as well as the reduction of adverse events (e.g. conjunctivitis) (Citation10, Citation11). However, no study analyzed predictive clinical and demographic factors affecting patient selection for dose spacing or treatment withdrawal due to satisfactory response.
In this study, we sought to define the profile of AD patients who successfully experienced dose spacing or treatment withdrawal, identifying those predictive factors helping tailor dupilumab treatment.
Materials and methods
This retrospective study included adult patients with moderate-to-severe AD, referring to the dermatology outpatient clinics at the Fondazione Policlinico Universitario A. Gemelli – University of the Sacred Heart in Rome, Italy, and at the University of Verona, from December 2018 to October 2022.
All patients treated with dupilumab for at least 16 weeks were considered. All patients were encouraged to use emollients daily, while topical corticosteroids or topical calcineurin inhibitors were applied as needed. The following clinical and demographic data were collected from patient charts: sex, personal history of AD or/and other atopic manifestation, age at AD onset, total IgE serum levels, clinical phenotypes (Citation13), topographical distribution of skin lesions, disease duration, comorbidities, previous and current therapies, age at treatment initiation, schedule of dupilumab administration. Disease severity was assessed by: a) Eczema Area Severity Index (EASI) varying from 0 to 72; b) itch Numeric Rating Scale (itch-NRS) ranging from 0 to 10; and c) Dermatology Life Quality Index (DLQI) varying from 0 to 30. Disease severity in patients affected by prurigo nodularis was assessed by a dedicated IGA score (Citation14).
Visits were performed at baseline, week 4, week 16, and every 16 weeks thereafter as regular schedule. Based on treatment response dose spacing was performed, extending of one or two weeks the dosing interval, and eventually leading to treatment interruption. Patients withdrawing dupilumab treatment and still referring to their dermatology center were followed-up. Safety was assessed by physical examination and eventual laboratory tests. Adverse events (AEs) were defined as any abnormal physical condition or blood test alteration, collected by the physicians throughout the study period every 16 weeks or more tightly based on clinical needs.
This study data collection was approved by the local ethical committees (Prot. N. 0046558/20).
Statistical analyses
Descriptive statistics was performed to analyze demographic and clinical variables. Categorical variables were described as frequencies and proportion, while continuous variables as mean ± standard deviation. Data were reported and analyzed “as observed” and, thereby, no missing imputation was performed. Comparisons of AD scores and clinical features at specified time points among the overall population subgrouping as standard schedule and dose spacing or withdrawal for satisfactory response cohorts were assessed by Student’s t-test. Logistic regression models were designed to evaluate the association between dose spacing or treatment withdrawal for satisfactory response and clinical predictor variables, non-cutaneous atopic manifestations, age at AD onset, age at treatment initiation (dichotomized as lower and higher to the median age distribution in our cohort), clinical phenotypes, topographical distribution of skin lesions, disease duration, previous and current therapies, baseline EASI and achievement of 90% EASI reduction from baseline [EASI90] to week 16 as meant of sustained treatment response). Variables that in the univariate analysis showed a p value <0.2, as well as variables we want to adjust their effects for, have been included in the multivariable models. Multicollinearity of the independent variables in the binary logistic regression have been assessed by VIF score.
Statistical analysis was performed using the Stata/BE statistical package version 17 (StataCorp., Texas, USA). Results were considered statistically significant with a p-value of <0.05.
Results
Therapeutic response to dupilumab in the overall study population
A total of 818 adult patients with moderate-to-severe AD (432 [52.8%] males; 386 [47.2%] females; mean age ± SD: 46.32 ± 21.3 years) with at least 16 weeks of dupilumab treatment was included in this study (). Most of them (89.5%) were affected by flexural AD phenotype, with head/neck involvement occurring in 67.1% of patients. History of rhinitis and asthma were reported in 36.2% and 21.3% of patients, respectively. Prior to dupilumab therapy, most patients underwent at least one systemic treatment with oral corticosteroids and cyclosporin being the most commonly prescribed.
Table 1. Clinical and demographic characteristics of the study population.
All patients were initially treated with dupilumab every two weeks at the dosage of 300 mg, after an initial 600 mg dose. A marked reduction of EASI, DLQI and itch-NRS scores was observed from baseline to week 16 and maintained thereafter ().
Table 2. Treatment response.
Overall, 146 patients (17.8%) withdrew dupilumab therapy due to satisfactory response (67/146, 45.9%), inefficacy (53/146, 36.3%), lost to follow-up (22/146, 15.1%), ocular adverse events (3/146, 2%) or the occurrence of other diseases (namely, one case of multiple uterine adenomatous polyps and ovarian cysts, 0.7%).
Clinical features of patients experiencing dose spacing or withdrawal for satisfactory response
Dose spacing or treatment withdrawal because of a satisfactory response was performed in 164/818 (20%) patients. In 8.2% (67/818) of cases, treatment withdrawal occurred due to a satisfactory response. In 11.9% (97/818) of patients, the regular dose interval was expanded beyond 2 weeks (14 days) by a mean additional period of 8.1 days, planning dupilumab administration every 3 weeks in 7.9% (65/818) of patients, and every 4 weeks in 3.9% (32/818) of patients. At the time of spacing or treatment withdrawal mean EASI score resulted of 2.6 ± 3.9, mean DLQI was 1.9 ± 3.0, with 68.9% (113/164) of patients achieving DLQI values of 0 or 1. None of the patients extended the dose interval because of safety reasons (e.g. to improve conjunctivitis), though we observed a clinical improvement of conjunctivitis in one patient performing dose spacing. The dose spacing was performed after a mean duration of the standard dose treatment of 68.6 ± 38.6 weeks and continued for a mean period of 75.5 weeks (± 28.5).
Treatment response was maintained after dose spacing or treatment withdrawal (). Overall, 21 patients (12.8%, 21/164) returned to the standard schedule or restarted dupilumab therapy. The resume of standard dose schedule was performed in 8 patients (8/164, 4.9%) due to AD recurrence in six cases, increased itching in one case, while one patient in the dose spacing group interrupted dupilumab because of the occurrence of ocular adverse events (namely conjunctivitis). In addition, 13 patients (13/164, 7.9%) restarted dupilumab after treatment withdrawal of 48 weeks (2 patients), 96 weeks (8 patients) or 144 weeks (3 patients).
describes disease severity (meant as mean EASI score, mean DLQI and mean itch-NRS values) and treatment response at the follow-up visits in the two subcohorts of patients identifying those treated with the standard dose schedule (654/818) and those experiencing dose spacing/withdrawal for satisfactory response (164/818). Patients with dose spacing or treatment withdrawal showed a superior response during the standard dose schedule, prior to performing dose spacing or treatment withdrawal, compared with those continuously treated with standard dose schedule ( and ).
Figure 1. Trends in mean EASI score over time during treatment with dupilumab, highlighting statistically significant difference between standard schedule and dose spacing or withdrawal due to remission groups.
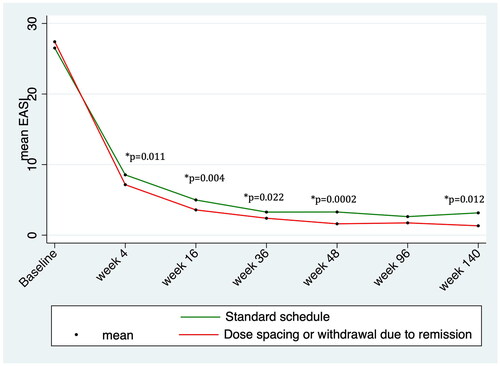
We analyzed the clinical and demographic features of patients with dose spacing or withdrawal for satisfactory response (), revealing a significantly higher age both at the time of treatment initiation and at the disease onset in patients with dose spacing or treatment withdrawal compared with standard dose schedule group (51.4 ± 21.0 versus 44.7 ± 0.9, p = 0.0004; 31.5 ± 27.9 versus 23.9 ± 26.5, p = 0.0018, respectively). Similarly, prurigo nodularis-like phenotype and the presence of atopic comorbidities were significantly more frequent in patients experiencing dose spacing or treatment withdrawal compared with the standard dose schedule group ().
Table 3. Clinical features of the study population and of its subgrouping into standard schedule, dose spacing and withdrawal for satisfactory response.
We sought to identify predictive factors of dose spacing or treatment withdrawal due to satisfactory response by multivariable logistic regression models design (). By adding variables that in the univariable analysis were significantly associated with the possibility of extending the dose interval (Supplementary Table 1), the multivariable logistic regression model identified the presence of non-cutaneous atopic manifestations, prurigo nodularis phenotype and the age at treatment initiation as the strongest predictors of dose spacing or treatment withdrawal while maintaining dupilumab effectiveness (Supplementary Table 2). The likelihood of dose spacing or treatment withdrawal for satisfactory response increased by 59% (p = 0.024) in the presence of non-cutaneous atopic manifestations (rhinitis, conjunctivitis, asthma), and by 80% (p = 0.015) for patients with age at treatment initiation higher than 44 years [median age distribution: 44 (IQR: 26–62)]. Prurigo nodularis compared with flexural phenotype was associated with a 3.5-fold increased likelihood for dose spacing (p = 0.001).
On the contrary, a sustained response (at least EASI 90 response at week 16), age at disease onset, previous systemic treatment and sex did not appear to influence the possibility of extending the dose interval during dupilumab treatment.
Defining the profile of patients with dose spacing vs. withdrawing treatment
We further dissected two sub-cohorts of patients: those with dose spacing vs. patients withdrawing therapy for satisfactory response (), revealing that patients withdrawing therapy showed a significant association with the prurigo nodularis-like phenotype (p = 0.008), a significantly higher age at treatment initiation (59.6 ± 22.3 vs. 45.8 ± 18.2, p < 0.0001) and age at disease onset (40.7 ± 30.2 vs. 25.3 ± 24.4, p = 0.0004), in particular a very late onset was more frequently associated with treatment withdrawal for satisfactory response (p = 0.003).
The multivariate logistic regression analysis (Supplementary Tables 3, 4) confirmed prurigo nodularis-like phenotype as the strongest predictor of treatment withdrawal (OR = 4.7; 95% CI =1.28–17.2; p = 0.020; Supplementary Table 4), while the presence of non-cutaneous atopic manifestation showed significance as predictor of dose spacing (OR = 0.48; 95% CI = 0.24–0.98; p = 0.044; Supplementary Table 4) irrespective of age at onset, sex and age at treatment initiation.
Discussion
This study confirmed that dupilumab effectiveness can be maintained in a subset of patients treated with longer dupilumab dosing intervals. A satisfactory response led to dose spacing or treatment withdrawal in 20% of cases, after a mean period of standard dose interval of more than one-year treatment. Notably, only a small portion (12.8%, 21/164) of them returned to the standard schedule or restarted dupilumab therapy due to AD recurrence.
In our study, the satisfactory response consisted of a mean EASI score of 2.6 ± 3.9, mean DLQI of 2.6 ± 3.9, with 68.9% (113/164) of patients achieving DLQI values of 0 or 1, according to the assessment prior to dose spacing or treatment withdrawal. These levels of disease activity can be easily controlled with topical drugs. The mean EASI value we detected prior to dose spacing or treatment withdrawal is in line with the EASI cutoff (≤7) maintained for at least 6 months, that was identified as criteria to include patients in the arms testing dupilumab dose spacing in a previous trial (Citation8). Notably, a large majority of patients with dose spacing or treatment withdrawal achieved a DLQI 0–1, indicating no impact on quality of life.
As previously observed, in a subset of dupilumab-treated patients dose spacing was associated with sustained treatment response and/or improvement of AEs, such as conjunctivitis (Citation8–12), though in our study the improvement of AEs was not the reason of expanding dose interval. In our study population, dose spacing or treatment withdrawal was performed to reduce treatment burden and reduce drug costs in those patients showing a prolonged and sustained satisfactory response over time. Dose spacing was mostly performed as a 3-week interval in 7.9% (65/818) while in 3.9% (32/818) of patients as a 4-week interval. The other subcohort of patients, consisting of 8.2% (67/818) of cases, withdrew treatment maintaining the therapeutic response, consistently with a real-world experience reporting a sustained treatment response after dupilumab suspension (Citation15).
During the previous period of standard dose schedule, the patient subcohort with dose spacing or treatment withdrawal showed a superior response with a significantly lower mean absolute EASI score, mean DLQI and mean itch-NRS values, observed as early as week 4 and throughout the various time points, compared with those continuing with the standard dose schedule. Notwithstanding patients with dose spacing or treatment withdrawal experienced a greater response compared with the standard dose schedule subcohort, an early satisfactory response (meant as the achievement of at least 90% improvement of the baseline EASI score [EASI 90] at week 16) did not appear to influence the likelihood of extending the dose interval during dupilumab treatment. The multivariable logistic regression models showed that the presence of non-cutaneous atopic manifestations, prurigo nodularis-like phenotype and the age at treatment initiation were the strongest predictors of dose spacing or treatment withdrawal while maintaining a minimal disease activity. Prurigo nodularis compared with flexural phenotype was associated with a about 3.5-fold increased likelihood for dose spacing (p = 0.001). Because non-cutaneous atopic manifestations and prurigo nodularis (characterized by an enhanced type 2 inflammatory signal), were associated with an overall superior response to treatment and they also resulted positive predictive factors for dose spacing and/or treatment withdrawal, a prominent role of type 2 inflammation in those patients may be suggested (Citation14, Citation16–18).
We also sought to identify any difference between the subset of patients performing dose spacing and the subset of patients withdrawing treatment due to satisfactory response: patients with very late-onset as well as prurigo nodularis-like phenotype and the age at treatment initiation (these two latter was also the strongest predictor for treatment withdrawal) were more frequently associated with treatment withdrawal, whereas the presence of current or past non-cutaneous atopic manifestations was more frequent in the dose spacing group vs. treatment withdrawal group, also confirmed as predictor factor for dose spacing.
Similar to our study (87.2%), a real-world prospective report described a successful dose tapering strategy with dupilumab in 83.3% (334/401) of patients who, in most cases, received dupilumab every 3 or 4 weeks, with only a slight increase of mean EASI (from 2.5 to 3.5) and itch NRS score (from 2.4 to 3.2) observed after the start of dose tapering (Citation12). In their experience the interval prolongation was performed in patient under treatment with dupilumab for at least one year and under disease control (EASI ≤ 7) for at least 6 months. No clinical or biological prognostic factors for successful tapering were identified in the multivariate analysis (Citation12). A further meaningful observation deriving from this Dutch experience was the estimated large cost saving through dose tapering, amounting to EUR 3,977,033.98 for 401 patients, between 10 January 2019 and June 2022 (Citation12).
Our study has some limitations, including the non-randomized design and the absence of a predefined control group, and the non-inclusion of biomarkers (i.e. dupilumab trough levels and serum concentrations of IL-4 and IL-13) that could facilitate the identification of patients who would benefit of dose spacing or treatment withdrawal and the time of starting dose interval extension. In addition, being a retrospective study, a potential bias selection, lack of generalizability, and other confounding factors such as the concomitant use of topical medications (corticosteroids and calcineurin inhibitors) that were permitted as needed (the amount and frequency of use of these drugs was not evaluated) could limit data interpretation.
However, the study also has some strengths such as the large number of patients involved, the real-word nature with the absence of pre-selection of patients.
In conclusion, our study confirmed in the majority of patients the maintenance of a good clinical response after dose spacing or discontinuation of dupilumab in the majority of patients, with the exception of 12.8% of subjects who returned to the regular dose schedule due to AD relapse. Notably, this study represents the first real-world experience identifying predictive factors of dose spacing or treatment withdrawal, in particular non-skin atopic manifestations, prurigo nodularis phenotype and age at treatment onset were the strongest predictors of dose tapering or drug discontinuation while maintaining minimal disease activity.
The identification of patients who would benefit of dose spacing or treatment withdrawal may be of interest for reducing a non-therapeutic drug exposure, improving patient perception of treatment burden and chronicity, and decreasing direct drug costs.
Author contributions
All authors made substantial contribution to this manuscript and provided revision and approval of the final draft for submission, in details: AC, GDB: Concept and design, analysis and interpretation of data, drafted the article. NG, GC, CDS, MM, DS: patient management, reviewed the article critically for important intellectual content. LDN: performed statistical analysis of data with graphical and tabular conceptualization. GDB, GC, NG: acquisition of data. KP, GG: reviewed the article critically for important intellectual content, gave final approval of the version to be published.
Ethics statement
We confirm that all the subjects gave their written informed consent and the study protocol was reviewed and approved by Fondazione Policlinico Universitario Agostino Gemelli IRCCS - Università Cattolica del Sacro Cuore, Prot N.: 0046558/20.
Supplemental Material
Download PDF (106.4 KB)Disclosure statement
Ketty Peris has served on advisory board, received honoraria for lectures and/or research grants for Abbvie, Almirall, Lilly, Galderma, Leo Pharma, Pierre Fabre, Novartis, Sanofi, Sun Pharma, Janssen. Clara De Simone has acted as a speaker and consultant for Almirall, AbbVie, Janssen, Celgene, Leo Pharma, Novartis, Eli Lilly, and UCB Pharma. Andrea Chiricozzi has served as advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Bristol Myers Squibb, Leo Pharma, Lilly, Janssen, Novartis, Pfizer and Sanofi Genzyme. Giacomo Caldarola has received honoraria as speaker and consultant for Abbvie, Almirall, Biogen, Eli Lilly, LEO Pharma, Novartis, Janssen, Sanofi, Pfizer, and UCB Pharma outside the submitted work. Giampiero Girolomoni served as consultant and/or speaker for AbbVie, Abiogen, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli-Lilly, Leo Pharma, Merck Serono, Novartis, Pfizer, Samsung bioepis, Sanofi and UCB pharma. Niccolò Gori served as consultant and/or speaker for AbbVie, Leo Pharma, and Sanofi. Giacomo Dal Bello, Donatella Schena, Lucia Di Nardo and Martina Maurelli have no conflict of interests to disclose.
Data availability statement
Enquiries related to the data generated or analyzed during this study can be directed to the corresponding author.
Additional information
Funding
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–6. doi: 10.1111/jdv.18345.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. doi: 10.1111/jdv.18429.
- D’Erme AM, Romanelli M, Chiricozzi A. Spotlight on dupilumab in the treatment of atopic dermatitis: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:1473–1480. doi: 10.2147/DDDT.S113192.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1.
- Fargnoli MC, Esposito M, Ferrucci S, et al. A 48-week update of a multicentre real-life experience of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J Dermatolog Treat. 2020;3:1–4.
- de Wijs LEM, Bosma AL, Erler NS, et al. Effectiveness of dupilumab treatment in 95 patients with atopic dermatitis: daily practice data. Br J Dermatol. 2020;182(2):418–426. doi: 10.1111/bjd.18749.
- Spekhorst LS, Bakker D, Drylewicz J, et al. Patient-centered dupilumab dosing regimen leads to successful dose reduction in persistently controlled atopic dermatitis. Allergy. 2022;77(11):3398–3407. doi: 10.1111/all.15439.
- Patruno C, Potestio L, Fabbrocini G, et al. Dupilumab dose spacing after initial successful treatment or adverse events in adult patients with atopic dermatitis: a retrospective analysis. Dermatol Ther. 2022;35(12):e15933. doi: 10.1111/dth.15933.
- Jendoubi F, Shourik J, Seneschal J, et al. Longer dupilumab dosing intervals in adult patients with atopic dermatitis: experience from a french multicentre retrospective cohort study. Br J Dermatol. 2022;187(4):602–603. doi: 10.1111/bjd.21628.
- Worm M, Simpson EL, Thaçi D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(2):131–143. doi: 10.1001/jamadermatol.2019.3617.
- Spekhorst LS, Boesjes CM, Loman L, et al. Successful tapering of dupilumab in atopic dermatitis patients with low disease activity: a large pragmatic daily practice study from the BioDay registry. Br J Dermatol. 2023;ljad159. doi: 10.1093/bjd/ljad159.
- Silvestre Salvador JF, Romero-Pérez D, Encabo-Durán B. Atopic dermatitis in adults: a diagnostic challenge. J Investig Allergol Clin Immunol. 2017;27(2):78–88. doi: 10.18176/jiaci.0138.
- Chiricozzi A, Maurelli M, Gori N, et al. Dupilumab improves clinical manifestations, symptoms, and quality of life in adult patients with chronic nodular prurigo. J Am Acad Dermatol. 2020;83(1):39–45. doi: 10.1016/j.jaad.2020.03.049.
- Chiricozzi A, Di Nardo L, Talamonti M, et al. Patients withdrawing dupilumab monotherapy for COVID-19-Related reasons showed similar disease course compared with patients continuing dupilumab therapy. Dermatitis. 2022;33(3):e25–e29. doi: 10.1097/DER.0000000000000814.
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21(1):135–136. doi: 10.1684/ejd.2010.1196.
- Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol. 2011;165(5):990–996. doi: 10.1111/j.1365-2133.2011.10498.x.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29(5):1180–1190. doi: 10.1038/s41591-023-02320-9.

