Dear Editor,
Roflumilast is a selective phosphodiesterase-4 (PDE-4) inhibitor approved for the treatment of chronic obstructive pulmonary disease (COPD) (Citation1), which may have a therapeutic role for diverse dermatologic conditions.
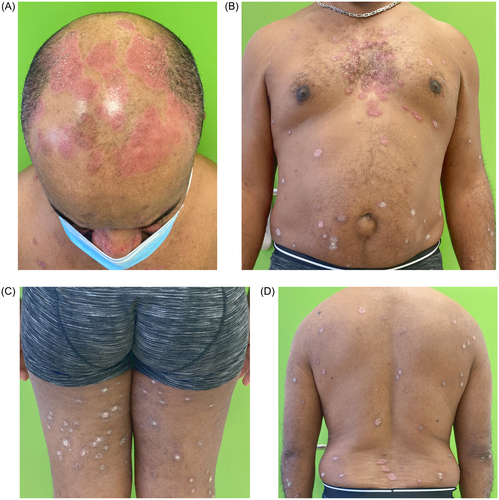
A 49-year-old man with no previous medical history other than obesity, was referred to the dermatology outpatient clinic for a 6-month history of skin lesions. Examination revealed erythematous and scaly plaques predominantly on the scalp and face, with particular involvement of the interciliary region, as well as the trunk and extremities. The latter were not pruritic, and the patient denied any systemic symptoms. Fungal culture was negative, and a clinical diagnosis of psoriasis was made. The total affected body surface area was 14%, while the Psoriasis Area and Severity Index (PASI) was 12.2, and the Physician’s Global Assessment (PGA) was 3 (moderate). Daily treatment was started with clobetasol foam (0.05%) on the scalp, fluticasone cream (0.05%) on the face, and betamethasone salicylic dipropionate ointment (0.05% + 2%) on the body. Blood tests revealed no relevant findings except for positive Interferon-Gamma Release Assays (IGRAs) for Mycobacterium tuberculosis (TBC). Active TBC was ruled out, and the patient refused treatment for latent TBC infection at that time.
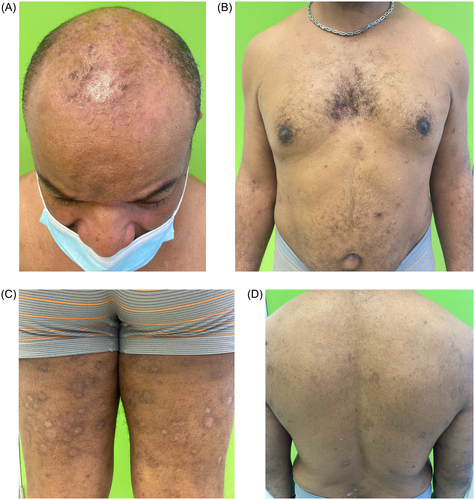
After one month, no improvement was observed (), so systemic alternatives were suggested. Given the profile of the patient (obesity) and lack of access to apremilast, interleukin-17 or interleukin-23 inhibitors (which are not accepted in our hospital in without prior systemic treatment), oral roflumilast 500 μg per day was started after obtaining written consent and explaining possible side effects. After two months of treatment, the psoriatic plaques had completely cleared except for post-inflammatory hyperpigmentation, and no new lesions were observed on examination (). Total affected body surface area and PASI were both 0. The patient wished to discontinue treatment due to clinical improvement. He also reported weight loss of up to 5 kg (albeit coincided with stressful life events), but did not experience nausea or vomiting while taking the medication. The patient was referred to the infectious diseases department for latent tuberculosis management.
Discussion
Oral roflumilast, a selective phosphodiesterase-4 (PDE-4) inhibitor, is approved for the treatment of chronic obstructive pulmonary disease (COPD) (Citation1). In experimental studies, PDE-4 activity was elevated in psoriasis lesions (Citation2). Roflumilast has a favorable safety profile and has the potential to improve not only skin manifestations but also psoriatic arthritis. No laboratory monitoring is required (Citation2). When roflumilast is used in combination with isoniazid in both acute and chronic murine models of tuberculosis, a decrease in the pulmonary bacillary burden is observed; consequently, roflumilast may be a suitable therapeutic alternative in patients with possible tuberculosis (Citation3).
Oral roflumilast has been used in diverse skin diseases, including psoriasis (), suppurative hidradenitis (4) or aphthosis (Citation5) with apparently good clinical outcomes, good tolerability and safety. It may also be beneficial in comorbidities such as metabolic syndrome or COPD (Citation6–9). In a recent clinical trial of oral roflumilast for the treatment of moderate to severe psoriasis, oral roflumilast (500 μg once daily) was effective and safe over 24 weeks (Citation10). A topical presentation of roflumilast is approved by the U.S. Food and Drug Administration (FDA), for the treatment of mild to moderate plaque psoriasis and intertriginous psoriasis, with a clearing rate of almost 45% at week 52 (Citation11).
Table 1. Previously reported cases of psoriasis treated with oral roflumilast.
Oral roflumilast was rapidly effective for our patient with psoriasis who was refractory to topical therapy. On the basis of the limited evidence available to date, this drug may also be suitable for psoriasis patients with latent tuberculosis. Oral roflumilast can be an easy-to-prescribe alternative in cases where access to other more expensive therapies is limited.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not applicable – no new data generated.
Additional information
Funding
References
- Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1–3. doi: 10.2147/COPD.S89849.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis. 2021;11:21–29. doi: 10.2147/ptt.s303634.
- Maiga MC, Ahidjo BA, Maiga M, et al. Roflumilast, a type 4 phosphodiesterase inhibitor, shows promising adjunctive, host-directed therapeutic activity in a mouse model of tuberculosis. Antimicrob Agents Chemother. 2015;59(12):7888–7890. doi: 10.1128/AAC.02145-15.
- Ring HC, Egeberg A, Zachariae C, et al. Considerable improvement in hidradenitis suppurativa with oral roflumilast therapy. Br J Dermatol. 2022;187(5):813–815. doi: 10.1111/bjd.21744.
- Gyldenløve M, Meteran H, Zachariae C, et al. Rapid improvement of idiopathic aphthous ulcers with oral roflumilast therapy. Br J Dermatol. 2022;187(2):258–259. doi: 10.1111/bjd.21044.
- Gyldenløve M, Meteran H, Zachariae C, et al. Long-term clearance of severe plaque psoriasis with oral roflumilast. J Eur Acad Dermatol Venereol. 2023;37(3):e429–e430. doi: 10.1111/jdv.18647.
- Egeberg A, Meteran H, Gyldenløve M, et al. Complete clearance of severe plaque psoriasis with 24 weeks of oral roflumilast therapy. Br J Dermatol. 2021;185(6):1251–1252. doi: 10.1111/bjd.20602.
- Gyldenløve M, Egeberg A. Killing all the birds with one drug–is oral roflumilast a novel treatment option for psoriasis? J Dermatolog Treat. 2022;33(6):2782–2783. doi: 10.1080/09546634.2022.2069223.
- Michels K, Hagner M, El Zein M, et al. Treating 2 diseases with 1 drug: PDE-4 inhibitor for COPD and psoriasis. Am J Ther. 2017;24(1):e103–e104. doi: 10.1097/MJT.0000000000000465.
- Gyldenløve M, Meteran H, Sørensen JA, et al. Efficacy and safety of oral roflumilast for moderate-to-severe psoriasis—a randomized controlled trial (PSORRO). Lancet Reg Health Eur. 2023;30:100639. doi: 10.1016/j.lanepe.2023.100639.
- Pixley JN, Schaetzle T, Feldman SR. A review of topical roflumilast for the treatment of plaque psoriasis. Ann Pharmacother. 2023;57(8):966–969. doi: 10.1177/10600280221137750.