Dear Editor,
Psoriasis is a chronic inflammatory skin disease that affects up to 3% of the general population worldwide (Citation1). The development of anti-Interleukin (IL) drugs has changed the management of moderate-to-severe plaque psoriasis, allowing patients to achieve complete or almost complete skin clearance with a very high safety profile (Citation2,Citation3). However, in patients who have had previous contact with Mycobacterium tuberculosis (tuberculosis (TB) infection), the treatment with biologics may increase the risk of tuberculosis reactivation (TB disease) (Citation4). While anti-Tumor Necrosis Factor (TNF)-alfa drugs should be avoided in these patients, there are very limited data on the use of IL-23 inhibitors (guselkumab, tildrakizumab and risankizumab) in patients with TB infection. A recent review on the safety of Il-17 inhibitors in patients with TB infection has shown a good safety profile of these drugs in this subpopulation (Citation5).
We conducted a real-life retrospective study to evaluate the effectiveness and safety of anti-IL-23 therapies in patients with moderate-to-severe plaque psoriasis and previous TB infection without evidence of TB disease (negative chest X-ray and absence of clinical signs or symptoms). We identified 16 patients from the Dermatology Unit of our Hospital (), all with a QuantiFERON-TB Gold positive test before the start of the biological treatment. In accordance with the pulmonologist, ten patients (62.5%) were treated with isoniazid for six months before receiving the first dose of anti-IL-23 drugs. The other six patients (37.5%) started an IL-23 inhibitor without prophylaxis after the pulmonologist ruled out active TB disease. The most commonly prescribed anti-IL-23 drug was risankizumab (11 patients), followed by guselkumab (3) and tildrakizumab (2). Each drug was administered according to its specific summary of product characteristics. Thirteen patients were male (81.3%), and the median age was 63.5 years, with an interquartile range (IQR) of 19.3. The median follow-up time was 18.8 months (IQR = 19.5). At baseline, the median PASI (Psoriasis Area and Severity Index) was 12 (IQR = 5.5). All patients received an IL-23 inhibitor in monotherapy, and they reached at least a 75% reduction in PASI score at the last available observation, with ten patients (62.5%) achieving complete skin clearance (). During follow-up, each patient underwent a chest X-ray every year. None of the patients showed evidence of TB reactivation throughout the study. No serious adverse events were observed in our experience. To date, all patients are still on treatment with the biological drug.
Figure 1. Percentage of PASI score reduction at the last available visit of our 16 patients. Blue bars: patients treated with risankizumab; Green bars: patients treated with gulsekumab; Purple bars: patients treated with tildrakizumab. PASI: Psoriasis Area and Severity Index.
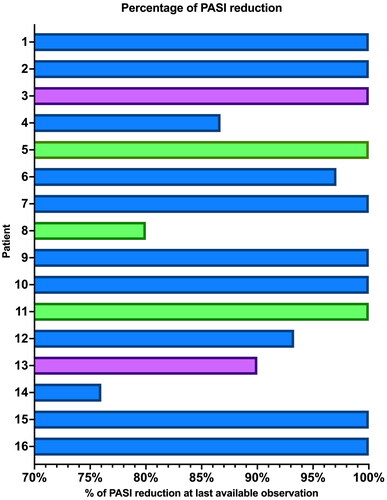
Table 1. Demographic and clinical characteristics of our patients.
The development of biological therapies for the treatment of moderate-to-severe psoriasis has made it necessary to highlight the safety profile of these drugs, even in patients with chronic infections (Citation6). There are limited data on the safety of these drugs in patients with tuberculosis infection. Most of these studies focused on anti-TNF-alfa drugs and included patients with rheumatic diseases (Citation4). Regarding IL inhibitors, preclinical studies have shown that the cytokines IL-23 and IL-17 may play a role against Mycobacterium tuberculosis (Citation7). However, recent evidence from both clinical trials and real-life experiences suggests that the role of these cytokines in eliciting this response may not be as important as previously thought (Citation8,Citation9). Based on a safety analysis of the phase-III clinical trials, VOYAGE 1 and VOYAGE 2, it was found that none of the 69 patients who received guselkumab and had tuberculosis infection experienced disease reactivation (Citation10). In a pooled analysis that included four phase-III clinical trials of risankizumab, none of the 72 patients who received TB prophylaxis for TB infection developed active TB during a mean follow‐up of 61 weeks (Citation11). In the IMMhance study, 31 patients who tested positive for QuantiFERON-TB at baseline did not receive prophylactic treatment and did not experience reactivation of tuberculosis after 55 weeks of treatment with risankizumab (Citation12). According to a recent real-life study by Mastorino et al. (Citation8), which mainly included patients treated with anti-IL-17 drugs, none of the two patients with TB infection who received risankizumab experienced reactivation of the disease after a mean follow-up of 9.3 months. Regarding tildrakizumab, a recent multicenter real-life study showed no signs of TB reactivation in six patients with TB infection after a year of treatment (Citation3). Our study supports the safety and effectiveness of IL-23 inhibitors for the treatment of moderate-to-severe psoriasis in patients with previous TB infection, with one of the longest mean follow-up to date.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. The patient received biologics as in good clinical practice, in accordance with European guidelines. The patient had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
L. Gargiulo has been a consultant for Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1–3. doi:10.1016/S0140-6736(20)32549-6.
- Gargiulo L, Ibba L, Malagoli P, et al. Real-life effectiveness and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: a 104-week multicenter retrospective study - IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol. 2023;37(5):1017–1027. doi:10.1111/jdv.18913.
- Narcisi A, Valenti M, Gargiulo L, et al. Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J Eur Acad Dermatol Venereol. 2023;37(1):93–103. doi:10.1111/jdv.18594.
- Souto A, Maneiro JR, Salgado E, et al. Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatology (Oxford). 2014;53(10):1872–1885. doi:10.1093/rheumatology/keu172.
- Fowler E, Ghamrawi RI, Ghiam N, et al. Risk of tuberculosis reactivation during interleukin-17 inhibitor therapy for psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2020;34(7):1449–1456. doi:10.1111/jdv.16254.
- Gargiulo L, Pavia G, Valenti M, et al. Safety of biologic therapies in patients with moderate-to-Severe plaque psoriasis and concomitant viral hepatitis: a monocentric retrospective study. Dermatol Ther (Heidelb). 2022;12(5):1263–1270. doi:10.1007/s13555-022-00726-w.
- Mourik BC, Lubberts E, de Steenwinkel JEM, et al. Interactions between type 1 interferons and the Th17 response in tuberculosis: lessons learned from autoimmune diseases. Front Immunol. 2017;8:294. Published 2017 Apr5. doi:10.3389/fimmu.2017.00294.
- Mastorino L, Dapavo P, Trunfio M, et al. Risk of reactivation of latent tuberculosis in psoriasis patients on biologic therapies: a retrospective cohort from a tertiary care Centre in Northern Italy. Acta Derm Venereol. 2022;102:adv00821. Published 2022 Nov 29. doi:10.2340/actadv.v102.1982.
- Nogueira M, Warren RB, Torres T. Risk of tuberculosis reactivation with interleukin (IL)-17 and IL-23 inhibitors in psoriasis - time for a paradigm change. J Eur Acad Dermatol Venereol. 2021;35(4):824–834. doi:10.1111/jdv.16866.
- Puig L, Tsai TF, Bhutani T, et al. Safety in moderate-to-severe plaque psoriasis patients with latent tuberculosis treated with guselkumab and anti-tuberculosis treatments concomitantly: results from pooled phase 3 VOYAGE 1 & VOYAGE 2 trials. J Eur Acad Dermatol Venereol. 2020;34(8):1744–1749. doi:10.1111/jdv.16460.
- Gordon KB, Lebwohl M, Papp KA, et al. Long-term safety of risankizumab from 17 clinical trials in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2022;186(3):466–475. doi:10.1111/bjd.20818.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658. doi:10.1001/jamadermatol.2020.0723.

