Dear Editor,
Atopic dermatitis (AD) is an inflammatory skin condition that afflicts millions worldwide, resulting in chronic pruritus that affects both children and adults (Citation1). Dupilumab is a monoclonal antibody that targets the IL-4Rα-subunit of the IL-4 and IL-13 receptor, thereby reducing type 2 inflammation. Real-world studies have documented the safety and efficacy of dupilumab for AD patients (Citation2). However, some individuals exhibit improvement in trunk and limb skin lesions, but experience suboptimal improvement in facial and neck skin lesions after receiving dupilumab treatment (Citation3,Citation4). Upadacitinib, a selective small-molecule inhibitor of Janus kinase 1 (JAK1), has been recommended for the treatment of moderate-to-severe AD (Citation5). Herein, we present a case series of six AD patients (3 male and 3 female; mean age: 18.67 ± 7.7 years, range 12-35 years) who received upadacitinib treatment for 12 weeks following an initial 14-20 weeks of dupilumab treatment without significant improvement in their facial and neck skin lesions (). Upadacitinib was administered at a once-daily dose of 15 mg to six AD patients. The effectiveness of the upadacitinib treatment was evaluated by measuring Eczema Area Severity Index (EASI, range: 0-72), Dermatology Life Quality Index (DLQI, range: 0-30), Atopic Dermatitis control tool (ADCT, range: 0-24), and Pruritus Numerical Rating Scale (P-NRS, range: 0-10) scores at baseline, week 4, week 8, and week 12. Besides, levels of pro-inflammatory cytokines (interleukin (IL)-4, IL-5, IL-13, IL-17, IL-22, IL-31, and IFN-ƴ), total IgE, and peripheral blood eosinophils were performed at baseline and after 12 weeks of upadacitinib treatment. Safety assessments included monitoring adverse events and conducting laboratory assessments such as blood count, liver and kidney function, lactate dehydrogenase and creatine phosphokinase, serum creatinine, and lipid status tests every 4 weeks. Continuous variables were given as mean ± standard error. Baseline characteristics were analyzed by t-test, and analysis of variance (ANOVA) using SPSS version 26.0, with a significance level of < 0.05.
Table 1. Clinical data of six AD cases.
These patients had a mean AD disease course of 12.5 ± 3.5 years. Only one (16.7%) patient had a personal atopic history of allergic rhinitis. After 14-20 weeks of treatment with dupilumab, the skin lesions on the trunk and limbs of the patients noticeably subsided, leaving only erythema, papules, and itching on the face and neck (). At baseline (prior to upadacitinib treatment), the mean EASI, DLQI, ADCT, and P-NRS scores were 8.1 ± 0.66, 12.5 ± 6.24, 11.5 ± 3.15, and 6.83 ± 0.37, respectively. A statistically significant improvement in EASI score was observed at week 4 (3.95 ± 0.75), week 8 (1.9 ± 0.89), and week 12 (0.73 ± 0.56) compared with baseline (p< 0.001). At week 12, all patients achieved EASI 75, and three (50%) even reached EASI 90. In addition, the DLQI, ADCT, and P-NRS scores of all patients showed significant improvement from baseline to 12 weeks after upadacitinib treatment (p< 0.001). The number of eosinophil in peripheral blood also significantly decreased from baseline (2.65 ± 3.24) to week 12 (1.42 ± 2.42). After 12 weeks of upadacitinib treatment, biomarkers of AD, including serum levels of IL-4, and IL-31 levels decreased, whereas total IgE, serum levels of IL-5, IL-13, IL-22, and IFN-ƴ remained unchanged and serum levels of IL-17 increased (). No patients reported adverse events during the treatment period.
Figure 1. (A, B) Face and neck lesions before treatment with dupilumab. (C, D) no significant improvement in the skin lesions of the face and neck after 12 weeks of dupilumab treatment. (E, F) Clinical improvement after 4 weeks of upadacitinib. (G, H) Marked improvement with clearing of face and neck lesions after 8 weeks of upadacitinib. (I, J) Clinical complete resolution after 12 weeks with upadacitinib without recurrence.
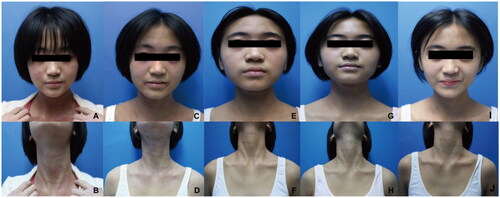
Table 2. Summary of the treatment efficacy of upadacitinib after 12 weeks of treatment.
Based on current literature and clinical practice, it is evident that dupilumab is effective in treating Atopic Dermatitis (Citation2,Citation5). However, some reports suggest that in some adolescents and adults with AD, the use of Dupilumab may not improve or may exacerbate head and neck dermatitis (Citation4,Citation6). Therefore, our study aims to explore the clinical effectiveness of upadacitinib treatment in AD patients with facial and neck skin lesions who have not responded to dupilumab treatment. One study reached similar conclusion using upadacitinib on dupilumab-associated head and neck dermatitis, with a significant reduction of EASI scores, total IgE and Malassezia-specific IgE. However, our study observed no significant difference in total serum IgE levels after 12 weeks of upadacitinib treatment when compared to baseline levels. Nonetheless, the study revealed a decrease in eosinophil count and serum levels of Interleukin-31 (IL-31) and Interleukin-4 (IL-4). These findings suggest that eosinophils may play critical roles in AD rash and itch symptoms by secreting IL-4/IL-13 or IL-31, which bind to their respective receptors on sensory nerve endings, IL-4Rα or IL-31RA, and activate the JAK1/STAT pathway (Citation7). Upadacitinib may suppress the effects of IL-31 or IL-4/IL-13 and block the communication between nerves and eosinophils, reducing pruritus. In addition, dual blockade of IL-4/IL-13 may lead to Th1/Th17 skewing (Citation8), thus contributing to the increase in serum IL-17 levels observed after 12 weeks of upadacitinib treatment. An increase in Th17 cytokines’ expression may induce a psoriasiform reaction pattern (Citation9) and promote Demodex colonization, leading to the development of rosacea (Citation10). However, the exact mechanism of action of upadacitinib in treating AD is still unclear and requires further extensive prospective studies to confirm. In conclusion, upadacitinib may be a promising option for AD patients with recalcitrant facial and neck dermatitis who do not respond to dupilumab. The dynamic changes observed in serum levels of IL-4 and IL-31 and eosinophils may serve as useful biomarkers to evaluate upadacitinib’s efficacy for treating AD.
Department of Dermatology, Guangzhou Institute of Dermatology, Guangzhou, Guangdong, China
#Yan Yang and Jiaoquan Chen have contributed equally to the [email protected]
Ethics statement
The patients in this manuscript have given written informed consent to the publication of their case details.
Data available statement
Data available on request from the authors.
Disclosure statement
None.
Additional information
Funding
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z.
- Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician. 2020;101(10):590–3.
- Nakanishi M, Tamagawa-Mineoka R, Arakawa Y, et al. Dupilumab-resistant facial erythema - Dermoscopic, histological and clinical findings of three patients. Allergol Int. 2021;70(1):156–158. doi:10.1016/j.alit.2020.07.001.
- Ahn J, Lee DH, Na CH, et al. Facial erythema in patients with atopic dermatitis treated with dupilumab - a descriptive study of morphology and aetiology. J Eur Acad Dermatol Venereol. 2022;36(11):2140–2152. doi:10.1111/jdv.18327.
- Wollenberg A, Christen-Zach S, Taieb A, et al. ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. doi:10.1111/jdv.16892.
- Soria A, Du-Thanh A, Seneschal J, et al. Development or exacerbation of head and neck dermatitis in patients treated for atopic dermatitis with dupilumab. JAMA Dermatol. 2019;155(11):1312–1315. doi:10.1001/jamadermatol.2019.2613.
- Kunsleben N, Rudrich U, Gehring M, et al. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Invest Dermatol. 2015;135(7):1908–1911. doi:10.1038/jid.2015.106.
- Bridgewood C, Wittmann M, Macleod T, et al. T helper 2 IL-4/IL-13 dual blockade with dupilumab is linked to some emergent T helper 17–type diseases, including seronegative arthritis and enthesitis/enthesopathy, but not to humoral autoimmune diseases. J Invest Dermatol. 2022;142(10):2660–2667. doi:10.1016/j.jid.2022.03.013.
- Bridgewood C, Sharif K, Freeston J, et al. Regulation of entheseal IL-23 expression by IL-4 and IL-13 as an explanation for arthropathy development under dupilumab therapy. Rheumatology (Oxford). 2021;60(5):2461–2466. doi:10.1093/rheumatology/keaa568.
- de Bruin-Weller M, Graham N, Pirozzi G, et al. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with demodex and increased interleukin-17 levels?: reply from the authors. Br J Dermatol. 2018;178(5):1220–1221. doi:10.1111/bjd.16348.

