Dear Editor,
Atopic dermatitis (AD) is a chronic debilitating inflammatory skin disease of fluctuating course, with a lifetime prevalence of up to 20% (Citation1). Although AD still has an unmet need for treatments, the last decade has brought a revolution in the pursuit of disease control, with the appearance of biologic drugs and Janus Kinase (JAK) inhibitors (Citation2).
Upadacitinib is a selective oral JAK-1 inhibitor that has demonstrated short and long-term efficacy and a safety profile in clinical trials (Citation3,Citation4). As a newly approved drug for adults and adolescents with moderate-to-severe AD, there are still many gaps in our knowledge about its use in daily practice. Should we keep upadacitinib for life in responding patients? In case of secondary failure, can retreatment be considered? If upadacitinib monotherapy is not enough to control flares, could it be combined with a biologic drug? We present three different scenarios focusing on upadacitinib retreatment in patients with severe AD, which to our knowledge have not been reported before.
Case 1 is a 43-year-old woman who had been treated with upadacitinib 30 mg daily and had achieved a score of 0 on the Eczema Area Severyty Index (EASI), Body Surface Area (BSA) and Worst-Itch Numerical Rating Scale (WI-NRS). After 2.5 years of sustained complete response, it was decided to discontinue upadacitinib. Three months after withdrawal the patient relapsed, presenting with generalized eczema (EASI 21, BSA 40%) and severe pruritus (WI-NRS 9). Upadacitinib 30 mg was reintroduced reaching complete response in 4 weeks, and after 8 months of follow-up she has had no relapses or adverse effects.
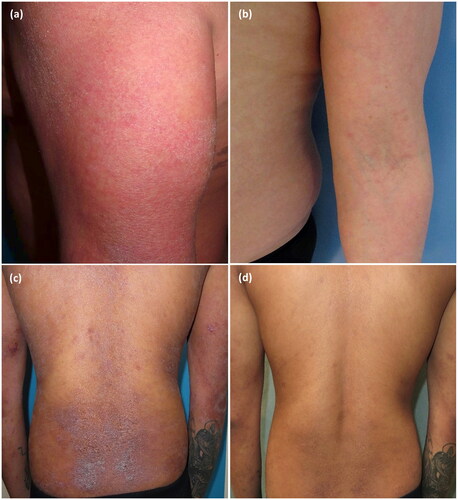
Cases 2 and 3 are two men, aged 42 and 22 respectively, who had been treated in the past with upadacitinib 30 mg daily, with a secondary loss of effectiveness after a complete response maintained for 1.5 years in case 2 and for 2 years in case 3. Both patients were subsequently treated with a biologic drug (tralokinumab in case 2 and dupilumab in case 3, both 600 mg loading dose and 300 mg every 2 weeks), with an insufficient response after 6 months of treatment. In case 2, upadacinitib 30 mg was reintroduced, achieving total control of pruritus and disappearance of eczema in 4 weeks (baseline EASI, BSA and WI-NRS of 31, 80% and 10, respectively). In case 3, dupilumab was maintained and upadacitinib 30 mg was reintroduced in combination, achieving a complete response after 8 weeks (baseline EASI, BSA, WI-NRS of 31, 80% and 10 respectively). After 5 months of follow-up, both cases have had no flares or adverse effects ().
Figure 1. (a) Case 2 after 6 months of treatment with tralokinumab with no response, prior to reintroduction of upadacitinib. (b) Case 2 after 5 months of retreatment with upadacitinib. (c) Case 3 after 6 months of treatment with dupilumab, prior to reintroduction of upadacitinib. (d) Case 3 after 5 months of retreatment with upadacitinib.
The effect of withdrawal and retreatment with upadacitinib was evaluated during a 32-week interim analysis from an 88-week phase 2b trial in patients with moderate-to-severe AD (Citation5,Citation6). Patients were randomized to receive upadacitinib (7.5, 15 or 30 mg) or placebo for 16 weeks. After this period, they were rerandomized, so that 53 patients who were being treated with upadacitinib began receiving placebo. In 4 weeks, 81% of the cases that had discontinued upadacitinib required its reintroduction, and most of these patients achieved an EASI reduction >75% after 8 weeks. Patients were able to recapture the initial response after withdrawal and retreatment, as in case 1. To our knowledge this upadacitinib intermittent treatment regimen has not been previously published in daily clinical practice.
There are also no case reports of loss of effectiveness of upadacitinib after initial good control and recovery of response after a rest period and reintroduction. Cases 2 and 3, with a secondary failure after 1.5 and 2 years respectively, and complete response after 4 and 8 weeks of retreatment, illustrate this scenario. Moreover, both cases had been previously unresponsive to a biologic drug. Some real-life case series have demonstrated the effectiveness of upadacitinib in patients with a prior failure on dupilumab, tralokinumab, or other JAK inhibitor; however, in no case was it a retreatment as in our cases (Citation7,Citation8).
Despite recent improvements in the AD therapeutic armamentarium, many challenges are still faced in the everyday clinical practice. JAK inhibitors are oral molecules and, at least from a theoretical point of view, its withdrawal and reintroduction would have a low risk of favoring immune responses against the drug.
To conclude, we report three different severe AD patients in which withdrawal and subsequent reintroduction of upadacitinib achieved a good disease control. Our observations need to be corroborated in the future, but we consider that they may be relevant when managing a disease with a chronic-recurrent course.
Disclosure statement
S. Martínez-Fernández has no competing interests to declare that are relevant to the content of this article.
HJ Suh-Oh has received honoraria or support for training activities, has acted as a consultant or has participated in clinical trials from: Abbvie, Celgene, Faes pharma, Isdin, Janssen, Leo-Pharma, Lethipharma, Lilly, Mylan, Novartis, Pfizer, Pierre Fabre, Roche y Sanofi-Genzyme.
A. Batalla has received honoraria or support for training activities, has acted as a consultant or has participated in clinical trials from: Abbvie, Celgene, Faes pharma, Isdin, Janssen, Leo-Pharma, Lethipharma, Lilly, Mylan, Novartis, Pfizer, Pierre Fabre y Sanofi-Genzyme.
C. Couselo-Rodríguez has received honoraria or support for training activities, has acted as a consultant or has participated in clinical trials from: Mylan, Pierre Fabre y Sanofi-Genzyme.
M. Espasandín-Arias has received honoraria or support for training activities, has acted as a consultant or has participated in clinical trials from: Abbvie, Sanofi, Pfizer and LEO Pharma.
Á. Flórez has received honoraria or support for training activities, has acted as a speaker, consultant or has participated in clinical trials from Abbvie, Almirall, Amgen, Celgene, Janssen, Kyowa Kirin, Leo-Pharma, Lilly, Novartis, Pfizer, Roche Farma, Sanofi, Sun Pharma, Takeda and UCB Pharma.
Data availability statement
The data that support the findings of this study are openly available in Pubmed at:
http://doi.org/10.1038/s41572-018-0001-z, reference number 1.
http://doi.org/10.1038/s41573-021-00266-6, reference number 2.
http://doi.org/10.1016/S0140-6736(21)00588-2, reference number 3.
http://doi.org/10.1001/jamadermatol.2022.0029, reference number 4.
http://doi.org/10.1016/j.jaci.2019.11.025, reference number 6.
http://doi.org/10.1007/s40268-022-00396-1, reference number 7.
http://doi.org/10.2340/actadv.v103.5243, reference number 8.
Additional information
Funding
References
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z.
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–3. doi:10.1038/s41573-021-00266-6.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/S0140-6736(21)00588-2.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi:10.1001/jamadermatol.2022.0029.
- Guttman-Yassky E, Beck LA, Anderson JK, et al. Upadacitinib treatment withdrawal and retreatment in patientswith moderate-to-severe atopic dermatitis from a phase 2b, randomized, controlled trial. Poster Presented at 2019 American Academy of Dermatology Annual Meeting; p. 1–5. 2019 Mar.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi:10.1016/j.jaci.2019.11.025.
- Chiricozzi A, Gori N, Narcisi A, et al. Effectiveness and safety of upadacitinib in the treatment of Moderate-Severe atopic dermatitis: a multicentric, prospective, Real-World, cohort study. Drugs R D. 2022;22(3):245–252. doi:10.1007/s40268-022-00396-1.
- Boesjes CM, Van der Gang LF, Zuithoff NPA, et al. Effectiveness of upadacitinib in patients with atopic dermatitis including those with inadequate response to dupilumab and/or baricitinib: results from the BioDay registry. Acta Derm Venereol. 2023;103:adv00872. doi:10.2340/actadv.v103.5243.

