Abstract
Abrocitinib, a highly selective inhibitor of Janus kinase 1 (JAK1), has been approved for the treatment of moderate-to-severe atopic dermatitis (AD). Patients with alopecia universalis (AU) co-morbid with AD receiving abrocitinib achieved clinical remission for both diseases. We report a case of a patient with AU after drug reaction with eosinophilia and systemic symptoms (DRESS) who responded well to abrocitinib therapy at a dose of 100 and 200 mg once daily. In addition, we reviewed cases of alopecia after DRESS and explored the underlying mechanisms for alopecia areata (AA) being an autoimmune sequela. The therapeutic effects of JAK inhibitors for AA may involve downstream cytokines, such as IFN-γ and IL-15. Abrocitinib may be a promising therapeutic option for recalcitrant AU.
Abrocitinib, a highly selective inhibitor of Janus kinase 1 (JAK1), has been approved for the treatment of moderate-to-severe atopic dermatitis (AD) at a recommended dose of 100 or 200 mg once daily (Citation1). Patients with alopecia universalis (AU) and co-morbid AD receiving abrocitinib achieved clinical remission for both diseases (Citation2,Citation3). Here, we report a case of a patient with AU after drug reaction with eosinophilia and systemic symptoms (DRESS) which responded well to abrocitinib therapy.
We presented a 30-year-old woman with a history of bipolar disorder and DRESS after one-month administration of lamotrigine. Eosinophils were markedly elevated in serum with a maximum level of 1.02 × 109/L (with an average level of 0.54 × 109/L). The patient also suffered from hepatic insufficiency and herpesvirus reactivation which manifested as multiple elevated liver enzymes and positive IgG and IgM antibodies of Epstein-Barr virus. Methylprednisolone 80 mg intravenously daily for 16 days and 60 mg for 3 days was effective; the patient transitioned to oral corticosteroids and regularly tapered in our outpatient clinics without recurrences. The total duration of corticosteroid administration intravenously and orally was eight months.
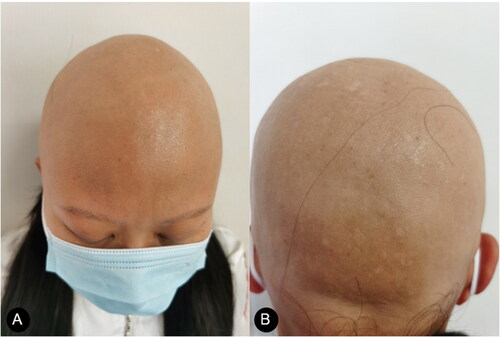
Two months after the first DRESS rash, the patient reported hair loss of the whole scalp. Intermittent external use of 2% minoxidil liniment was ineffective. At follow-up, she gradually suffered from full-body hair loss including eyebrows and eyelashes () and the shedding of fingernails and toenails (without pictures provided). AU was diagnosed as an autoimmune sequela from DRESS without any other autoimmune disorders such as autoimmune thyroiditis and systemic lupus erythematosus.
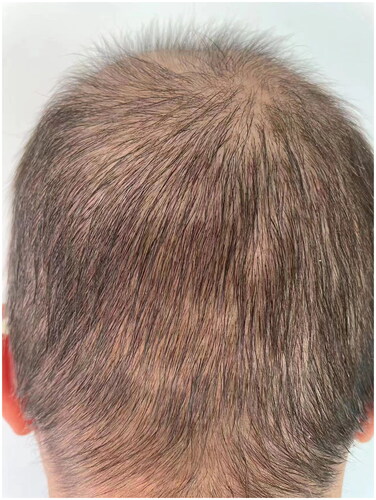
Compound betamethasone was injected intramuscularly once a month two times with minimal improvements. Then tofacitinib at a dose of 5 mg orally once a day for 2 months was introduced, and her hair loss continued. The patient was treated with abrocitinib (100 mg), administered orally once daily for 2 months. Regrowth of a patch of hair on the occiput was noted (). Abrocitinib was increased to 200 mg once daily for 2 months with great improvements and then tapered to 100 mg once daily; 6 months after the initiation of abrocitinib therapy, she experienced significant improvements as regrowth of terminal hairs on her scalp (). Meanwhile, no significant adverse effects were reported in clinical symptoms and laboratory tests. The patient then stopped taking medication and is currently undergoing follow-up. The limitation of our case was a lack of trichoscopy, histopathology, and data for long-term follow-up.
Figure 3. (A, B). Visible regrowth of terminal hairs on her scalp after six-month abrocitinib treatment.
Alopecia areata (AA) is an autoimmune disease with multiple etiopathogenetic factors. Total loss of all scalp and body hair is defined as AU. DRESS is a severe cutaneous drug reaction characterized by eruptions, fever, lymphadenopathy, hematologic abnormalities, and multisystem involvement. Thirteen cases, including this one, reported AA or AU or alopecia totalis as complications after DRESS. There were eight other cases demonstrating hair loss without a specific diagnosis after DRESS (Citation4,Citation5). The duration from DRESS to alopecia onset varied from weeks to months and most of them responded well to treatment. Herpesvirus reactivation and regulatory T cell dysfunction in resolution stage were considered to be involved in the development of long-term autoimmune complications including AA (Citation6). A loss of immune tolerance to hair follicles can lead to AA in DRESS patients (Citation4). Eosinophils detected in lesions of AA patients may be associated with the cause of the disease (Citation7). As eosinophilia being one of the typical clinical features of DRESS, there may be some underlying mechanistic links between these two diseases. We speculate that eosinophils play a role in DRESS-caused AA, which is still poorly understood. DRESS patients with complications of hair loss tended to be more severe (Citation4). AA is associated with mental stress and anxiety. Our patient was a young woman with a history of bipolar disorder and was very nervous and anxious during the course of DRESS. Hence, it cannot be ruled out that emotional factors were involved in the formation of AA.
JAK inhibitors, like tofacitinib, are effective for AA, presumably by downregulating cytokines, such as IFN-γ and IL-15, which are critical in the development of AA/AU (Citation7). In three cases, abrocitinib was administrated at a dose of 100 mg or 200 mg daily for the treatment of severe AD with AU with good efficacy for both (Citation2, Citation3). This broadens the spectrum of diseases that can be treated with abrocitinib. With AD being a risk factor of AA/AU, abrocitinib can achieve a therapeutic effect on AA/AU by inducing remission of the triggering factors. As patients receiving the pan-JAK inhibitor tofacitinib usually need more than a median of 14 months of treatment for good clinical response (Citation8), we cannot rule out the possibilities that the lower efficacy of tofacitinib in our patient was due to the short time it was used. Abrocitinib was rapidly effective, and we assume that highly selective JAK inhibitors like abrocitinib might be more effective than tofacitinib for AU with favorable safety profile. There was a case of lamotrigine-induced DRESS and post-DRESS alopecia similar to our patient which was refractory to topical and intralesional steroids and oral methotrexate (Citation9). Our case suggests that abrocitinib can be a promising therapeutic option for recalcitrant AU and also provides a new perspective on the role of JAK in the pathological mechanism of AU.
Acknowledgements
The authors thank National High Level Hospital Clinical Research Funding under Grant number 2022-PUMCH-B-092; and Beijing Municipal Natural Science Foundation under Grant number 7232118.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Deeks ED, Duggan S. Abrocitinib: first approval. Drugs. 2021;81(18):1–3. doi:10.1007/s40265-021-01638-3.
- Zhao J, Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. 2022;22:99–100. doi:10.1016/j.jdcr.2022.02.027.
- Bennett M, Moussa A, Sinclair R. Successful treatment of chronic severe alopecia areata with abrocitinib. Australas J Dermatol. 2022;63(2):274–276. doi:10.1111/ajd.13836.
- Lee JW, Yu DA, Cho S, et al. Hair loss after drug reaction with eosinophilia and systemic symptoms (DRESS): a multicentric retrospective case series. J Dermatol. 2023;50:814–819.
- Krivda LK, Campagna LJ, Mignano MS, et al. Prolonged drug-induced hypersensitivity syndrome/DRESS with alopecia areata and autoimmune thyroiditis. Fed Pract. 2022;39(8):350–354.
- Aksoy H, Karadag AS, Turgut Erdemir VA, et al. Alopecia universalis following DRESS, where rarities merge. Dermatol Ther. 2021;34(2):e14842.
- Zhou C, Li X, Wang C, et al. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. 2021;61(3):403–423. doi:10.1007/s12016-021-08883-0.
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi:10.1016/j.jaad.2016.09.007.
- Hollingsworth P, Paci K, Evans M, et al. Alopecia universalis after drug reaction with eosinophilia and systemic symptoms (dress). Pediatr Dermatol. 2020;37(5):947–949. doi:10.1111/pde.14217.