Dear Editor
Lupus miliaris disseminatus faciei (LMDF) is an uncommon granulomatous skin disease featured by skin-colored or light-yellow smooth papules or nodules mainly on the central face, classically the lower eyelid. Perifollicular granulomas can be found on histology. Pathogenesis of LMDF is still uncertain. Although LMDF is self-limiting and its natural disease course lasts 12–24 months, it is still a cosmetic problem and early interventions may reduce scar formation [Citation1]. Currently, treatments including tetracycline, systemic steroids and dapsone don’t always show satisfactory efficacy.
Tofacitinib is an oral, small-molecule Janus kinase (JAK) inhibitor, preferentially inhibiting JAK1 and JAK3. It has been approved by the US Food and Drug Administration for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis [Citation2]. Since its outstanding therapeutic effect on inflammatory or autoimmune diseases, we speculate that tofacitinib may be useful in treating LMDF. Herein, we report two cases of good response of LMDF treated with tofacitinib.
Case 1
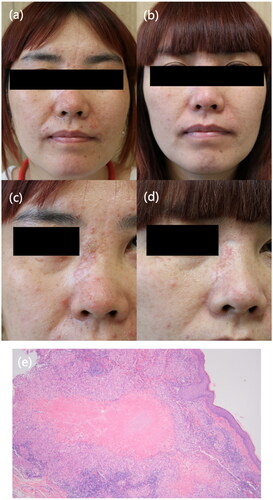
A 39-year-old female presented with a 6-month history of multiple reddish and yellowish papules on the face without systemic symptoms. Physical examination revealed symmetrically multiple monomorphic smooth reddish papules on her eyelids, cheeks, noses and chins. Skin biopsy results showed perifollicular epithelioid cell granulomas and lymphocytes infiltrating. Caseating granulomas were observed in the histopahtology of a biopsy specimen. A diagnosis of LMDF was made. She was treated with oral doxycycline at a dosage of 100 mg once daily plus hydroxychloroquine 200 mg twice daily with topical tacrolimus. However, there was no obvious improvement. We changed the therapeutic regimen to tofacitinib (Xeljanz) (5 mg twice daily) plus hydroxychloroquine for 2 months. At the 6-week follow-up, her lesions were improved greatly. Then she continued taking tofacitinib and these lesions improved gradually in the following 10 months ().
Figure 1. Clinical presentation of Patient 1. (a, c) Multiple erythematous and reddish papules on the patient’s face at baseline. (b, d) The lesions improved after oral tofacitinib treatment. (e) Routine pathology (hematoxylin eosin, original magnification ×20) showing caseating granulomas and lymphocytes infiltrating.
Case 2
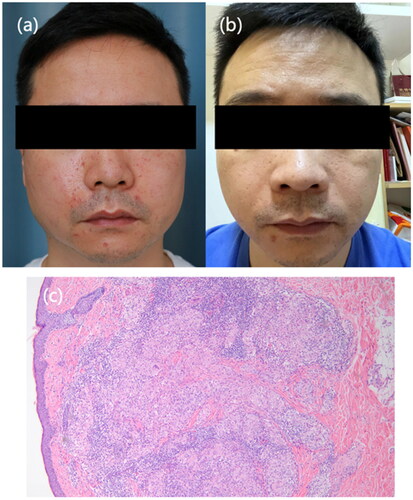
A 40-year-old male presented with multiple erythematous papules on the central part of his face on the face for 6 months. Non-caseating granulomas and lymphocytes infiltrating were observed in the specimen. He was treated with only oral tofacitinib (Xeljanz) (5 mg twice daily), and significant reduction in facial papules was observed half month later. The lesions disappeared gradually in the following one year as he kept taking tofacitinib ().
Figure 2. Clinical presentation of Patient 2. (a) Multiple erythematous papules on the central part of the patient’s face at baseline. (b) Nearly resolution of lesions after oral tofacitinib treatment. (c) Routin pathology (hematoxylin eosin, original magnification ×20) showing non-caseating granulomas and lymphocytes infiltrating.
Although LMDF is self-limiting, more effective treatment is needed to shorten the disease course and reduce the risk of scar formation. Oral JAK inhibitors have been reported successfully used in granulomatous diseases such as cutaneous sarcoidosis and generalized granuloma annulare [Citation3,Citation4]. Thus, we pustulate that tofacitinib which blocking JAK-STAT signal may be effective in treating LMDF. Very recently, a report revealed that topical JAK inhibitor-ruxolitinib cream is effective for LMDF [Citation5]. Our successful attempt shows that oral JAK inhibitors as monotherapy or in combination with other drugs are promising for LMDF. Studies with large sample are needed to reveal the efficacy and safety of oral JAK inhibitors for the treatment of LMDF.
Ethical approval
The two patients in this manuscript have given written informed consent.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Al-Mutairi N. Nosology and therapeutic options for lupus miliaris disseminatus faciei. J Dermatol. 2011;38(9):1–2. doi: 10.1111/j.1346-8138.2011.01244.x.
- Roskoski R.Jr. Properties of FDA-approved small molecule protein kinase inhibitors: a 2023 update. Pharmacol Res. 2023;187:106552. doi: 10.1016/j.phrs.2022.106552.
- Damsky W, Wang A, Kim DJ, et al. Inhibition of type 1 immunity with tofacitinib is associated with marked improvement in longstanding sarcoidosis. Nat Commun. 2022;13(1):3140. doi: 10.1038/s41467-022-30615-x.
- Damsky W, Thakral D, McGeary MK, et al. Janus kinase inhibition induces disease remission in cutaneous sarcoidosis and granuloma annulare. J Am Acad Dermatol. 2020;82(3):612–621. doi: 10.1016/j.jaad.2019.05.098.
- Gorham NC, Jacobs J, Wu SZ. Response of severe lupus miliaris disseminatus faciei to treatment with ruxolitinib cream. JAMA Dermatol. 2023;159(7):790–791. doi: 10.1001/jamadermatol.2023.0528.

