Abstract
Hand eczema is one of the most frequent dermatological diseases, with an incidence increased during the COVID-19 pandemic. The impact on life quality is considerable, giving rise to the need for a psycho-dermatological approach. This is a Randomized Control Trial (RCT) evaluating, either by the dermatological or psychological point of view, the effectiveness of an emollient and rehydrating topical product (Rilastil Difesa Sterile® cream) versus a standard treatment (i.e. moisturizing basic cream) in a group of 51 healthcare workers suffering from hand eczema during the COVID-19 pandemic. The enrolled subjects were randomized into a treatment or a control arm, treated for 8 weeks, and monitored through a clinical score (HECSI) and questionnaires evaluating the impact of the pathology and treatment on quality of life (DLQI and QOLHEQ). A psychometric evaluation was performed using the SCL-90 R, OCI-R, and CPDI scales. Our data, despite not reaching the statistical significance, demonstrated that both the clinical and psychological scores decreased mostly in patients treated with Rilastil Difesa Sterile® cream when compared to those treated with simple topical emollients. Moreover, we observed a high level of psychic suffering in dermatological patients and a parallel change in dermatological and psychological indicators, thus confirming their connection.
Introduction
Hand eczema (HE) is one of the most common and persistent dermatological disease with a relapsing course and variable duration (Citation1,Citation2). The prevalence of the chronic condition is difficult to estimate because many affected patients do not seek treatment (Citation3,Citation4). Indeed, HE represents 9-35% of the occupational diseases and affects 2-10% of the general population with a lifetime prevalence of 14.5% (Citation4–6), recognizing as main risk factors atopic dermatitis, contact allergy, exposure to irritants, wet work, and frequent hand washing (Citation5,Citation7,Citation8).
During the recent COVID-19 pandemic, the World Health Organization (WHO) and other health care authorities recommended frequent hand washing and disinfection to prevent the virus transmission (Citation9,Citation10). In this context, hand hygiene habits of the population have been changed both in the workplace and at home (Citation11–13). Especially, healthcare workers (HCWs) are exposed to wet work and irritants, and most of them had to dramatically change their hand hygiene routine; so, the prevalence of occupational dermatoses, such as HE, has increased during the pandemic (Citation11,Citation14,Citation15).
HE has a long-lasting negative impact on quality of life with an economic burden for the society (Citation16). The symptoms, such as itching and pain, cause sleep disorders and inability to participate in social activities involving the hands (Citation16,Citation17). Furthermore, when the HE is work-related, patients may sometimes have to change their job (Citation16). Clinical signs (scaling, redness, vesicles and hyperkeratosis) also contribute to negative esthetic modifications and in severe cases lead to embarrassment, social isolation and depression.
The negative impact of dermatological diseases on the patient’s quality of life led to the birth of the Psychodermatology, a recent field of study and research, resulting from the convergence of Psychiatry and Dermatology (Citation18,Citation19). It concerns the study of mind-skin connection (Citation19), focusing on their mutual interactions (Citation18,Citation19). The mostly used classification of psychodermatological disorders consists of four groups of conditions: i) primary psychopathology focused on the skin, ii) psychophysiological dermatoses, iii) cutaneous sensory disorders, iv) secondary psychiatric disorders (Citation19,Citation20). The ‘primary psychopathology focused on the skin’ group includes all the conditions in which a primary psychopathological disease may cause subtle skin lesions (Citation19,Citation20), as in the case of body dysmorphic disorder (Citation21). The ‘psychophysiological dermatoses’ include all the skin diseases that are stress-induced or stress-worsened, involving for example psoriasis and hyperidrosis (Citation19,Citation22) The ‘cutaneous sensory disorders’ are a cluster of abnormal skin sensations that have no evidence of skin alterations that may be responsible for the unpleasant sensation (Citation19). The last group is the group of ‘secondary psychiatric disorders’, also known as ‘disfiguring skin condition’, which includes the psychological consequences of a skin disease that has a severe impact on the body image of the patients and on their quality of life, for the severity of the illness or for its localization on the body (Citation19,Citation23,Citation24), such as atopic dermatitis or HE.
The objective of this study, was to evaluate in a group of HCWs suffering from HE during the COVID-19 pandemic in a North-Western Italy Hospital, the efficacy of a topical product with an emollient and rehydrating action (Rilastil Difesa Sterile® cream) versus a standard comparative product represented by a moisturizing basic cream. The possible impact on the quality of life and any improvement related to the treatment were also assessed through psychometric indicators.
Materials and methods
Study design
A prospective randomized controlled trial was conducted in June 2021 among students and employees of Maggiore della Carità Hospital, Novara, Italy. Subjects were recruited from a cross sectional study aimed to estimate the prevalence of HE among the HCWs in a North Italy Hospital after the third waves of COVID-19 pandemic. The methodology and the data of this survey are reported elsewhere (Citation25). Of 863 survey respondents, we considered only subjects who had at least three self-declared symptoms of HE among erythema, scaling, little vesicles, fissures and edema. Respondents without email or telephone number, with less than 18 years old and who did not give consent to be recalled for dermatological evaluation were excluded. Then, subjects were contacted, and a visit was performed (t0). If self-reported symptoms were confirmed, they were randomly assigned to a two treatment groups: Rilastil Difesa Sterile® cream vs comparative cream (moisturizing basic cream). Then patients were visited after 1 (t1) and 2 (t2) months of treatment. At each visit (t0, t1, t2) dermatological evaluation was done while information on psychological scores based on self-reported questionnaire were recorded only at t0 and t2.
Treatment
The product utilized in our study is a sterile cream particularly suitable for subjects with sensitive and reactive skin. Due to its protective, emollient, soothing and rehydrating action, it promotes the homeostasis of the skin barrier by reducing itching, burning and other discomfort and is indicated in case of intolerance to common cosmetic products and predisposition to allergic or eczematous manifestations. The cream is packaged in a P.P.A.T (Premium Protection Airless tube) with non-return valve to ensure sterility, high barrier and without metal parts.
The active ingredients and their characteristics are shown in . The cream was supplied free of charge by the pharmaceutical manufacturer to patients enrolled in this study.
Table 1. Composition of Rilastil Difesa sterile® cream.
The treatment provided to the control group was a simple moisturizing basic cream with the following ingredients: water, petrolatum, liquid paraffin, alcohols, stearic acid, urea, and parabens. Even the moisturizing basic cream was provided free of charge to the patients at the enrollment.
For each treatment arm the indication was to apply the product twice a day for two months.
Outcome
Dermatologic evaluations were performed using the following indicators: i) HECSI (Hand Eczema Severity Index), with score between 0 (clear) and ≥ 117 (very severe); ii) DLQI (Dermatology Life Quality Index), with score between 0 (no effect at all on patient’s life) and 30 (extremely large effect on patient’s life); iii) QOLHEQ (Quality Of Life in Hand Eczema Questionnaire), with score included <8 (no impairment) and >80 (very severe impairment) (Citation26–28).
Psychometric evaluation was performed using the following scales: i) Symptom Checklist-90 Revised (SCL-90 R); ii) Obsessive-Compulsive Inventory-Revised (OCI-R); iii) Covid-19 Peritraumatic Distress Index (CPDI).
SCL-90 R is a self-administered questionnaire exploring the severity of both internalizing (depression, somatization and anxiety) and externalizing (aggressiveness, hostility, impulsivity) symptoms over the previous week (Citation29). It includes nine subscales: Somatization (SOM); Obsessive-compulsive (O-C); Interpersonal sensitivity (I-S); Depression (DEP); Anxiety (ANX); Hostility (HOS); Phobic anxiety (PHOB); Paranoid ideation (PAR); and Psychoticism (PSY). There are seven additional items (OTHER) that explore disturbances in appetite and sleep (Citation29).
The OCI-R is a 18-items questionnaire, containing six subscales that explore the severity of typical obsessive symptoms: washing, checking, ordering, obsessing, hoarding, mental neutralizing (Citation30). The mean score of patients affected by OCD is 28; in the general population a score ≥ 21 is considered as a risk for OCD (Citation31).
The CPDI is a 24-items questionnaire used to evaluate the risk of developing a Post-Traumatic Stress Disease due to the Covid-19 pandemic (Citation32). The total score range is 0-96, and the final score is obtained dividing this score to 100: a final score <28 indicates no distress, scores from 28 to 51 represent mild to moderate distress, a score >51 stands for severe distress (Citation32).
Statistical analysis
Study data will be collected and managed using the REDCap electronic data capture tools hosted at Università del Piemonte Orientale.
A descriptive analysis was conducted considering subjects overall and separately for treatment group. Absolute and relative percentages were reported for categorical variables while mean and standard deviation or median and interquartile range for numerical ones, as appropriate.
To evaluate possible diversities in term of clinical and psychological outcome, differences among t1 and t0 (when data available) and t2 and t0, separated for treatment and control group were calculated and parametric and non-parametric tests were used. Then, the clinical scores were categorized based on literature, and we considered clinically significant improved only subjects who moving from worst score categories to higher ones. Moreover, for clinical scores, repeated measures models were performed to consider of all data available. Each clinical score was considered in a separate model as outcome and time and treatment were used as covariates.
Finally, the association between clinical improvement (considered in dichotomous way) and difference among psychological score was assessed using non-parametric test.
A p-value of 0.05 (two tails) was considered statistically significant and all the analysis were conducted using the Intention-to-treat approach. The software used was SAS 9.4.
Sample size and randomization
Participants will be blind randomly assigned to receive treatment vs control in a 1:1 ratio. An independent statistician generated the random allocation sequence with random block sizes of four and six patients and the list was integrated in the REDCap.
The primary objective of the study was to evaluate the difference of HECSI at t1 and t2 respect to t0. Assuming a medium difference (effect size = 0.4) between baseline and two months treatment among treated and control group, with a two tailed first type error of 0.05 and a power of 0.80 (with an intra-subject correlation coefficient of 0.90), at least 20 patients for groups were needed.
Results
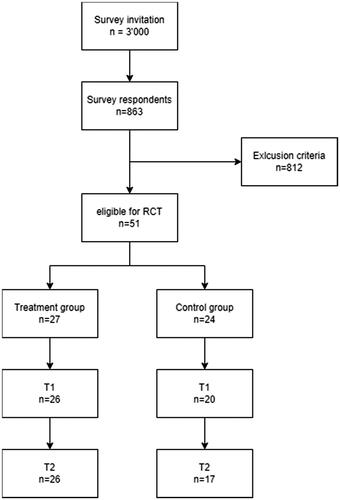
Of the 3000 subjects who were invited to participate, 863 (28.8%) completed the survey; 51 of these respected the inclusion criteria for this study. Of subjects enrolled in the RCT, 34 (66.67%) had three self-declared cutaneous HE signs among erythema, scaling, little vesicles, fissures, and edema, 10 (19.61%) had 4 signs, and the remaining 7 (13.73%) all five. Then, 27 subjects were randomly assigned to treatment and 24 to the control group ().
The sample was mainly composed of females (n = 41, 80.39%) and the mean age was 38.49 (SD 13.28) years. Most patients were medical doctors (n = 15, 29.41%) and nurses (n = 14, 27.45%) but only 27.45% (n = 14) of subjects worked in COVID-19 wards during the pandemic. The most frequent self-reported diseases related to HE were allergic rhinitis (n = 15, 29.41%), atopic dermatitis (n = 15, 29.41%), and contact skin allergies (n = 12, 23.53%), followed by allergic conjunctivitis (n = 8, 15.69%) and asthma (n = 7, 13.73%). Descriptive statistics are reported in . No statistical differences (p > 0.05) were observed among the groups, indicating that the randomization worked properly.
Table 2. Demographical and clinical data of subjects enrolled in RCT, separately for treatment group.
Dermatologic indices
The main dermatologic indices were evaluated (see ); the median values were 8 [IQR 5-22] for HECSI, 2 [IQR 1-6] for DLQI, 23 [IQR 11-48] for QOLHEQ. No statistically significant differences were observed among groups (p-value: HECSI 0.5730, DLQI 0.6021 and QOLHEQ 0.9401). In we reported a raw graphical representation of indices in time, separated for treatment.
Figure 2. Graphical representation of Measure of HECSI, DLQI and QOLHEQ for non-treated and treated subjects at T0, T1, T2. For HECSI, to better visualized the data we do not show the three data that were higher than 100 (outlier values): 2 for non-treated T0 and one for non-treated T1.

Table 3. Difference of median [IQR] values of dermatologic indices separated for non-treated and treated.
Generally, the dermatologic indicators decreased in time, and particularly the reduction was higher in T2 vs T0 than T1 vs T0. Moreover, treated subjects seem to have a higher improvement than non-treated ones, even if no statistical differences were observed ( and ).
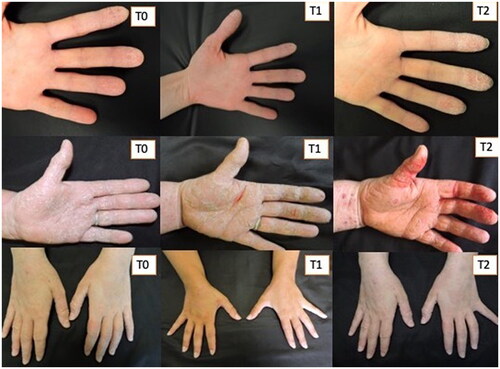
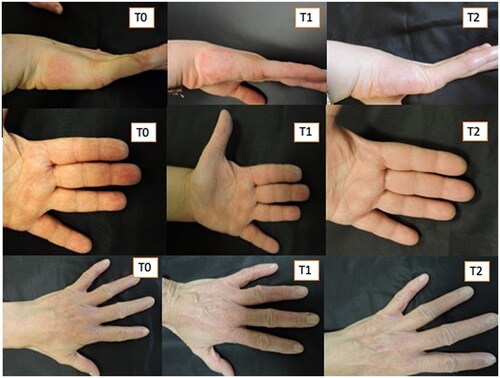
Figure 3. Three patients treated with Rilastil Difesa sterile®. improvement of erythema and scaling during the two months of treatment.
Then, considering the scores categorized, we observed that at baseline the majority of subjects had a HECSI almost clear (n = 37, 72.55%), 9 (17.65%) moderate, 3 (5.88%) severe, and 2 (3.92) very severe. The effect of disease on quality of life (DLQI) was absent for 20 (39.22%) patients, small for 15 (29.41%), moderate for 9 (17.65%) while for 5 (9.80%) and 2 (3.92%), the effect was very severe and extremely severe, respectively. Finally, slight, and moderate impairment was observed for 19 (37.25%) and 14 (27.45%) individuals, while 9 (17.65%) subjects had no impairment and 17.65% had severe (n = 7, 13.73%) or very severe (n = 2, 3.92%).
When comparison among times (T1 vs T0 and T2 vs T0) was conducted in term of score improvement, no statistically significant differences between groups were observed. Generally, the improvement was between 15% to 45% in T1 vs T0 and higher than 40% in T2 vs T0 ().
Table 4. Subjects who had an improvement: they moved from a worst to higher score categories.
Finally, the models that considered time and treatment confirmed the results reported above. The treatment seems associated with a decrease in dermatological scores in each model (-9.74 [95% CI −22.51; 3.03], −0.20 [95% CI −2.86; 2.46], and −3.03 [95% CI −14.59; 8.52] for HECSI, DLQI, QOLHEQ, respectively). However, no statistical significance was found between groups (p > 0.10). Moreover, the estimates suggested that the time had stronger and significant reduction in scores (). Median percentual variations are reported in supplementary materials (Table S1).
Table 5. Repeated measures models considering as outcome the dermatological scores (HECSI, DLQI, QOLHEC) and as covariate the time and the treatment.
Psychometric assessment
and report the results of the questionnaires at baseline and the difference between the median scores at T2 and T0.
Figure 5. Graphical representation of CPDI, OCI, and SCL-9 0 measures for non-treated and treated subjects in the two Temporal times (T0, T2). for SCL-90, to better visualize the data we did not show one datum for the non-treatment group that was higher than 200 (outlier values).

Table 6. Difference of median [IQR] values of psychological indicators separated for non-treated and treated patients.
At baseline a median value of 9 [IQR 2-15] was observed for CPDI, 4 [IQR 2-10] for OCI and 26.11 [IQR 4.44-55.55] for SCL-90. Generally, in the non-treated group we observed higher scores, though no statistically significant differences were recorded (p-value 0.0657, 0.3475, 0.2012).
Concerning the difference in time, in both groups there was a decrease in the psychometric tests scores: in the control group, CPDI was −5, OCI-R was −2.5, SCL-90 was −11.67, while in the Rilastil Difesa Sterile® cream group CPDI was 0, OCI-R was −0.5, SCL-90 was −4.44. The non-treated group showed a greater decrease in the questionnaire scores than the treated one, despite no statistical difference were found.
shows the association of dermatological (HECSI, DLQI, QOLHEQ test) improvement with the psychometric score (CPDI, OCI-R and SCL-90). Both in the successful and not successful group of the HECSI, DLQI and QOLHEQ there was a decrease of the CPDI, OCI-R and SCL-90 median scores; but seems that the decrease was greater in the group with a successful dermatological test. However, no significant differences were observed.
Table 7. Association among psychometric and dermatological questionnaires: dermatological score was considered in dichotomous way (improved vs non-improved) while psychometric one in numerical way.
Discussion
This paper aims to evaluate: i) the clinical efficacy of Rilastil Difesa Sterile® cream versus a standard treatment represented by a basic moisturizing cream and ii) the possible impact of this treatment on the quality of life in a group of HCWs affected by hand eczema after the third pandemic wave in a University Hospital of North-Western Italy.
In our cohort, the frequency of HCWs hand eczema increased from 38.1% in the pre-pandemic period to 51.1% in the post-COVID era, as reported in our previous study (Citation25) and following the literature (Citation15,Citation33–36), with a female preponderance (80.39%), as in most published case series (Citation9,Citation37–39). The majority of patients were medical doctors and nurses confirming the fact that not only post-COVID hand hygiene habits allow the development of hand eczema, but also the work activity itself, which in any case involves the use of gloves and exposure to irritating substances (Citation40). Furthermore, as reported in the literature (Citation40), in our series, the main comorbidities associated with hand eczema were atopic dermatitis and contact skin allergies.
The clinical characteristics of the group treated with the study product and those of the control group were superimposable. Also, the median values for dermatologic indices considered (HECSI, DLQI, and QOLHEQ) didn’t show statistically significant differences among treatment and control groups. Surprisingly, despite the location and persistence of the skin lesions clinically observed, their impact on the patient’s quality of life was relatively scarce: in fact, at enrollment, we observed relatively low scores in the DLQI questionnaire in most of the patients, and only 7 patients have a severe or very severe impact of the hand eczema on quality of life, with scores that decreased of 1 point during the treatment for each category.
These data are in agreement (Citation38) with those reported in the literature from Ibler et al. with a difference in DLQI after treatment of 0.78 points between the group of treatment and controls; in other studies, the maximum scores varied from 7 to 9 (Citation41,Citation42); for patients undergoing patch tests, the scores varied from 5 to 3 in case of a positive test and from 5 to 1.5 for patients with negative results. The impact of hand eczema on the patient’s quality of life, based on the DLQI, would appear to be low, and completely null after an intervention (Citation43).
More interesting are the data obtained from the use of the questionnaire QOLHEQ, specifically prepared to evaluate the impact of hand eczema on the quality of life and the patient’s satisfaction (Citation3,Citation37). In our experience, the baseline scores obtained using this questionnaire are higher, and the reduction after treatment is greater; moreover, the results in terms of scores reduction are in favor of Rilastil Difesa Sterile® cream.
In our study, the clinical evolution of the lesions was objectively quantified through the HECSI score.
We observed a greater reduction after treatment in the group of patients who applied Rilastil Difesa Sterile® cream, compared to the control group treated only with emollients, with an overall improvement of 9.74 points (treated vs non-treated – and ). On the other hand, Ibler et al. obtained an overall improvement of 3.10 in treated vs non-treated patients using unspecified moisturizers and protective gloves, indirectly confirming the greater efficacy of the product used in the present study. Indeed, Loden et al. had demonstrated that patients affected by hand eczema could delay relapses using a urea-containing cream. The median time to eczema-relapse was 20 days vs. 2 days, respectively, in the moisturizer and no treatment groups. In our study, we observed a constant improvement during the two months of cure for patients treated with with Rilastil Difesa Sterile® cream, so much more than the 20 days of delay of the recurrence with the use of a simple moisturizing cream.
To date, there is still a gap in literature regarding the association between psychometric and dermatological tests; however, it is known that skin lesions cause psychological suffering in those who carry them (Citation44,Citation45), and, therefore it can be expected that the improvement of the dermatological lesions corresponds to a consequent psychological improvement. This hypothesis was confirmed by our results. Nonetheless, some participants had pathological scores: the maximum score of the OCI-R was 48.00 in the control group and 52.00 in the Rilastil Difesa Sterile® cream group, both above the cutoff for OCD. The maximum CPDI score was 38.00 and 30.00 in the control and in the Rilastil Difesa Sterile® cream group, respectively, suggesting a mild degree of distress.
Concerning the T2-T0 differences in the psychometric questionnaires, all their scores decreased, even if no significant result was found in either of the two groups. We hypothesize that the inclusion in the study, regardless of the dermatological treatment received, probably allowed the patient to feel ‘cared for’ and therefore the concerns about their skin disease was alleviated.
Regarding the association between the psychometric test and the dermatological improvement, the group with a positive response at the HECSI, DLQI and QOLHEQ had a higher decrease in the median scores at the psychometric tests; the greatest difference can be observed with the HECSI test scores, corroborating our hypothesis that when the dermatological lesion improves there is an increase in mental well-being.
Our study suffers from some limitations: i) the monocentric design with a relatively small number of patients enrolled, ii) the topical treatment which can lead to poor adherence to therapy and therefore not provide realistic results, and iii) the self-administered psychological questionnaires. Its strengths are represented by the possibility to study a well-characterized sample with similar risk factors for hand eczema (i.e. a group of HCW during the COVID-19 pandemic) and by the innovative combined psycho-dermatological approach.
In conclusion the data obtained from this study, although not fully statistically significant either from a clinical or psychological point of view, allow us to highlight important consideration: i) Rilastil Difesa Sterile® cream seems to lead a greater improvement in patients suffering from hand eczema compared to simple topical emollients, both through a direct comparison with a control group and through an indirect comparison with literature studies; ii) dermatological and psychological indicators change in a parallel way, confirming the connection of these two aspects; iii) finally, the fact that there is already a high level of psychic suffering in dermatological patients indicates that more studies in this field are needed.
Supplemental Material
Download PDF (21.7 KB)Acknowledgments
The authors wish to thank the participants to the study. They also thank Ganassini Corporate for providing the product (Rilastil Difesa Sterile) evaluated in the study.
Disclosure statement statements
The authors declare no conflict of interest.
Data availability statement
The data will be available from the authors upon request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lodén M, Wirén K, Smerud KT, et al. Treatment with a barrier-strengthening moisturizer prevents relapse of hand eczema: an open, randomized, prospective, parallel group study. Acta Derm Venereol. 2010;90(6):1–8. doi:10.2340/00015555-0964.
- Meding B, Wrangsjo K, Jarvholm B. Fifteen-year follow-up of hand eczema: persistence and consequences. Br J Dermatol. 2005;152(5):975–980. doi:10.1111/j.1365-2133.2005.06494.x.
- Barrett A, Hahn J, Pedersen N, et al. Patient‑reported outcome measures in atopic dermatitis and chronic hand eczema in adults. Patient. 2019;12(5):445–459. ‑ doi:10.1007/s40271-019-00373-y.
- Elston DM, Ahmed DD, Watsky KL, et al. Hand dermatitis. J Am Acad Dermatol. 2002;47(2):291–299. doi:10.1067/mjd.2002.122757.
- Loh EDW, Yew YW. Hand hygiene and hand eczema: a systematic review and meta-analysis. Contact Dermatitis. 2022;87(4):303–314. doi:10.1111/cod.14133.
- Quaade AS, Simonsen AB, Halling A-S, et al. Prevalence, incidence, and severity of hand eczema in the general population – a systematic review and meta-analysis. Contact Dermatitis. 2021;84(6):361–374. doi:10.1111/cod.13804.
- Thyssen JP, Johansen JD, Linneberg A, et al. The epidemiology of hand eczema in the general population – prevalence and main findings*. Contact Dermatitis. 2010;62(2):75–87. doi:10.1111/j.1600-0536.2009.01669.x.
- Lerbaek A, Kyvik KO, Ravn H, et al. Incidence of hand eczema in a population-based twin cohort: genetic and environmental risk factors. Br J Dermatol. 2007;157(3):552–557. doi:10.1111/j.1365-2133.2007.08088.x.
- Techasatian L, Thaowandee W, Chaiyarit J, et al. Hand hygiene habits and prevalence of hand eczema During the COVID-19 pandemic. J Prim Care Community Health. 2021;volume12:21501327211018013. doi:10.1177/21501327211018013.
- Roshan R, Feroz AS, Rafique Z, et al. Rigorous hand hygiene practices among health care workers reduce hospital associated infections during the COVID-19 pandemic. J Prim Care Community Health. 2020;11:2150132720943331. doi:10.1177/2150132720943331.
- Symanzik C, Stasielowicz L, Brans R, et al. Prevention of occupational hand eczema in healthcare workers during the COVID-19 pandemic: acontrolled intervention study. Contact Dermatitis. 2022;87(6):500–510. doi:10.1111/cod.14206.
- Balato A, Ayala F, Bruze M, et al. European task force on contact dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34(8):e353–e354. doi:10.1111/jdv.16557.
- Metin N, Turan Ç, Utlu Z. Changes in dermatological complaints among healthcare professionals during the COVID-19 outbreak in Turkey. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29(3):115–122.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol. 2020;82(5):1215–1216. doi:10.1016/j.jaad.2020.03.014.
- Erdem Y, Altunay IK, Aksu Çerman A, et al. The risk of hand eczema in healthcare workers during the COVID-19 pandemic: do we need specific attention or prevention strategies? Contact Dermatitis. 2020;83(5):422–423. doi:10.1111/cod.13632.
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(Suppl. 1):4–12. doi:10.1111/jdv.16061.
- Moberg C, Alderling M, Meding B. Hand eczema and quality of life: a population-based study. Br J Dermatol. 2009;161(2):397–403. doi:10.1111/j.1365-2133.2009.09099.x.
- Jafferany M, Franca K. Psychodermatology: basics concepts. Acta Derm Venereol. 2016;96:35–37.
- Leon A, Levin EC, Koo JYM. Psychodermatology: an overview. Semin Cutan Med Surg. 2013;32(2):64–67. doi:10.12788/j.sder.0002.
- Jafferany M, Ferreira BR, Patel A. The essentials of psychodermatology. 1st ed. Berlin (Germany): Springer; 2020.
- Phillips KA, Hart AS, Simpson HB, et al. Delusional versus non delusional body dysmorphic disorder: Recommendations for DSM-5. CNS Spectr. 2014;19(1):10–20. doi:10.1017/S1092852913000266.
- Peters EMJ. Stressed skin? – A molecular psychosomatic update on stress-causes and effects in dermatologic diseases. J Dtsch Dermatol Ges. 2016;14(3):233–252; quiz 253. doi:10.1111/ddg.12957.
- Orion E, Wolf R. Psychologic consequences of facial dermatoses. Clin Dermatol. 2014;32(6):767–771. doi:10.1016/j.clindermatol.2014.02.016.
- Hawro M, Maurer M, Weller K, et al. Lesions on the back of hands and female gender predispose to stigmatization in patients with psoriasis. J Am Acad Dermatol. 2017;76(4):648–654.e2. doi:10.1016/j.jaad.2016.10.040.
- Veronese F, Esposto E, Airoldi C, et al. Prevalence of Self-Reported hand eczema signs among HealthcareWorkers after the ThirdWave of COVID-19 pandemic: a survey in a Northern Italy hospital. Medicina. 2023;59:1054. doi:10.3390/medicina59061054.
- Ofenloch RF, Weisshaar E, Dumke A-K, et al. The quality of life in hand eczema questionnaire (QOLHEQ): validation of the german version of a new disease-specific measure of quality of life for patients with hand eczema. Br J Dermatol. 2014;171(2):304–312. doi:10.1111/bjd.12819.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. A study of inter- and intraobserver reliability. Br J Dermatol. 2005;152(2):302–307. doi:10.1111/j.1365-2133.2004.06305.x.
- Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. lin. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x.
- Prunas A, Sarno I, Preti E, et al. Psychometric properties of the Italian version of the SCL-90-R: a study on a large community sample. Eur Psychiatry. 2012;27(8):591–597. doi:10.1016/j.eurpsy.2010.12.006.
- Sica C, Ghisi M, Altoè G, et al. The italian version of the obsessive compulsive inventory: its psychometric properties on community and clinical samples. J Anxiety Disord. 2009;23(2):204–211. doi:10.1016/j.janxdis.2008.07.001.
- Foa EB, Huppert JD, Leiberg S, et al. The obsessive-compulsive inventory: development and validation of a short version. Psychol Assess. 2002;14(4):485–496. doi:10.1037/1040-3590.14.4.485.
- Costantini A, Mazzotti E. Italian validation of CoViD-19 peritraumatic distress index and preliminary data in a sample of general population. Riv Psichiatr. 2020;55(3):145–151.
- Celik V, Ozkars MY. An overlooked risk for healthcare workers amid COVID-19: occupational hand eczema. North Clin. Istanb. 2020;18:527–533.
- Daye M, Temiz SA, Isic B, et al. Evaluation of the effect of COVID-19 pandemic on dermatological diseases with dermatological quality life index. Dermatol. Ther. 2020;33:e14368.
- Lampel H, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Contact Dermat. 2008;59:301–306.
- van der Meer EWC, Boot CRL, van der Gulden JWJ, et al. Hand eczema among healthcare professionals in The Netherlands: prevalence, absenteeism, and presenteeism. Contact Dermatitis. 2013;69(3):164–171. doi:10.1111/cod.12099.
- Politiek K, Ofenloch RF, Angelino MJ, et al. Quality of life, treatment satisfaction, and adherence to treatment in patients with vesicular hand eczema: a crosssectional study. Contact Dermatitis. 2020;82:201–210.
- Ibler KS, Jemec GBE, Diepgen TL, et al. Skin care education and individual counselling versus treatment as usual in healthcare workers with hand eczema: randomised clinical trial. BMJ. 2012;345:e7822. doi:10.1136/bmj.e7822.
- Ibler KS, Agner T, Hansen JL, et al. The hand eczema trial (HET): design of a randomised clinical trial of the effect of classification and individual counselling versus no intervention among health-care workers with hand eczema. BMC Dermatol. 2010;10:8. doi:10.1186/1471-5945-10-8.
- Guertler A, Moellhoff N, Schenck TL, et al. Onset of occupational hand eczema among healthcare workers during the SARS-CoV-2 pandemic: comparing a single surgical site with a COVID-19 intensive care unit. Contact Dermatitis. 2020;83(2):108–114. doi:10.1111/cod.13618.
- Agner T, Andersen KE, Brandao FM, et al. Hand eczema severity and quality of life: a cross-sectional, multicentre study of hand eczema patients. Contact Dermatitis. 2008;59(1):43–47. doi:10.1111/j.1600-0536.2008.01362.x.
- Wallenhammar LM, Nyfjall M, Lindberg M, et al. Health-related quality of life and hand eczema—a comparison of two instruments, including factor analysis. J Invest Dermatol. 2004;122(6):1381–1389. doi:10.1111/j.0022-202X.2004.22604.x.
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–664. doi:10.1111/j.0022-202X.2005.23621.x.
- van der Schaar WW, Lamberts H. [Scratching for the itch in eczema; a psychodermatologic approach]. Ned Tijdschr Geneeskd. 1997;141(43):2049–2051.
- Oska C, Nakamura M. Alternative psychotherapeutic approaches to the treatment of eczema. Clin Cosmet Investig Dermatol. 2022;15:2721–2735. doi:10.2147/CCID.S393290.