Abstract
Objective
Fixed-combination halobetasol propionate (0.01%) and tazarotene (0.045%) lotion (HP/TAZ) is approved for the treatment of plaque psoriasis in adults, with a demonstrated efficacy and safety profile in phase 3 trials. This study examined the effect of HP/TAZ on the reduction of tumor necrosis factor alpha (TNF-α) and interleukin 17 A (IL-17A) and its correlation to psoriasis improvement.
Materials and methods
Ten adults with mild-to-moderate plaque psoriasis and 2 symmetrical plaques self-applied HP/TAZ (treated plaque) or vehicle lotion (untreated plaque) for 12 weeks. At baseline and each study visit (weeks 2, 4, 8, and 12), Investigator’s Global Assessment (IGA) score and erythema, scaling, and induration were assessed. Additionally, D-squame tape strips were utilized to quantify TNF-α and IL-17A in target lesions by enzyme-linked immunosorbent assay.
Results
Significant improvements in mean IGA score in HP/TAZ–treated compared with untreated plaques were evident at week 2 and maintained through week 12 (p < 0.003). HP/TAZ significantly reduced TNF-α levels at weeks 4 through 12 (p < 0.03) and IL-17A levels at weeks 2 through 8 (p < 0.05) in treated compared with untreated plaques.
Conclusions
HP/TAZ was highly effective in treating psoriasis plaques and, although HP/TAZ is not a biologic, effectively reduced cytokine-associated inflammatory markers that drive psoriatic disease.
Keywords:
Introduction
Psoriasis is an immune-mediated inflammatory disease driven by cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 17 A (IL-17A) (Citation1,Citation2). TNF-α is derived from activated dendritic cells, keratinocytes, T helper 1 cells, and T helper 17 (Th17) cells, and elevated levels of TNF-α are found in psoriatic skin compared to the skin from healthy individuals (Citation3,Citation4). TNF-α amplifies inflammation in psoriasis through several pathways, including facilitating inflammatory cell entry into psoriasis lesions by inducing adhesion molecules on vascular endothelial cells, stimulating keratinocyte production of other pro-inflammatory cytokines, and activating dermal macrophages and dendritic cells (Citation5).
The IL-17 family plays a pivotal role in the pathogenesis of psoriasis (Citation6) via the release of pro-inflammatory cytokines (Citation7). Psoriasis patients have increased expression of IL-17A at sites of inflammation (Citation8), and the involvement of IL-17A in other autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and multiple sclerosis suggests a role in promoting autoimmune pathology (Citation7). TNF-α integrates with IL-17A mRNA, upregulating IL-17A signaling and simultaneously overexpressing IL-17 receptor on keratinocytes, causing keratinocyte hyperproliferation (Citation9); thus, targeting TNF-α and IL-17A in psoriasis patients has become an effective strategy to reduce disease burden.
Several approved anti-TNF-α biologics for moderate-to-severe psoriasis, including etanercept (Citation10), infliximab (Citation11), and adalimumab (Citation12), limit TNF-α from stimulating other immune components in psoriasis. Additionally, approved biologics for moderate-to-severe psoriasis that target IL-17A either selectively bind to IL-17A (secukinumab (Citation13), ixekizumab (Citation14)) or to IL-17A receptors (brodalumab (Citation15)). However, biologic dosing schedules may not be ideal and require invasive injections (Citation16). Although topical therapies are less invasive than are biologics, the ability of a topical therapeutic to downregulate inflammatory cytokines in psoriasis plaques, especially following treatment discontinuation, is not well studied. Additionally, rebound caused by cessation of topical steroids can result in worsening of psoriasis symptoms, highlighting the importance of determining the effects of topical therapy on inflammatory cytokine concentrations after therapy discontinuation (Citation17).
The use of topical corticosteroids is routine in the treatment of psoriasis; however, their continuous use is limited to 2–4 weeks because of long-term safety concerns (Citation18,Citation19). Tazarotene, a topical retinoid, has also shown efficacy in the treatment of psoriatic plaques, but its use is limited by skin irritation (Citation20). More recently, a novel fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% (HP/TAZ) lotion was evaluated in two phase 3 trials in subjects with moderate-to-severe psoriasis (Citation19). Subjects were randomized to receive HP/TAZ lotion or vehicle, which was applied to the affected area once daily for 8 weeks, with a follow-up posttreatment assessment at week 12. Treatment success was achieved in over 40% of subjects receiving HP/TAZ, with 33.3% experiencing a sustained therapeutic effect during the 4-week posttreatment period. In a long-term phase 3 study, 83.9% of participants who stopped therapy after achieving an IGA of 0 after week 8 did not require retreatment for >4 weeks, and 62.5% did not require retreatment for >8 weeks (Citation21–23). Additionally, HP/TAZ was well tolerated during the year-long study, with minimal safety concerns. However, the ability of HP/TAZ to reduce inflammatory cytokines in psoriatic plaques during and after treatment discontinuation is unknown.
In this study, we utilized D-squame real-time sampling to examine the effect of HP/TAZ lotion on reducing inflammatory cytokines and to determine whether such an anti-inflammatory effect correlates with psoriasis improvement.
Materials and methods
Study design
Key inclusion criteria included male and female subjects with mild-to-moderate plaque psoriasis aged 18 years or older, with 2 plaques suitable for tape stripping, and in general good health determined by medical history. Subjects with known allergies or sensitivities to ingredients in the test products, latex or adhesive allergies, pustular or erythrodermic psoriasis, and pregnant or nursing subjects were excluded. Exclusion criteria also included subjects currently enrolled in any other clinical study; subjects deemed by the investigator to be unable to complete the study; and the use of any other lotion, medication, or other topical product to the psoriasis plaques.
At the baseline visit, subjects underwent selection of 2 symmetrical target plaques by the investigating dermatologist. Plaques with an IGA score of mild (2) or moderate (3) qualified for inclusion. Plaques were also graded for erythema, scaling, and induration at baseline. One target plaque was designated as the HP/TAZ–treated plaque and the other target plaque as the untreated control. Subjects were instructed to self-apply HP/TAZ to the treated plaque once daily at bedtime.
At each subsequent study visit (weeks 2, 4, 8, and 12), plaques were photographed, and the study investigator graded plaques for IGA score, as well as erythema, scaling, and induration; also, at each visit, study participants self-assessed plaques for redness, thickness, and scaling. Subjects also underwent D-squame tape strip removal at each study visit on the 2 target plaques. Additionally, 10 D-squame tape strips were taken from a healthy individual without psoriasis to serve as a negative control. If subjects experienced complete plaque clearance in the treated plaque (IGA = 0) prior to week 12, HP/TAZ was discontinued and subjects returned for a final tape stripping at the next study visit.
A signed informed consent form was obtained from each subject prior to study procedures. No study-related procedures or activities were performed until each subject was fully informed and the consent form was signed and dated.
Efficacy endpoints
The primary endpoint was the decrease in TNF-α or IL-17A after achieving an IGA of 0 in HP/TAZ–treated plaques compared to untreated plaques. The secondary endpoint was the decrease, or maintained decrease, in TNF-α or IL-17A after 4 weeks of treatment in HP/TAZ–treated plaques compared to untreated plaques. Incidence of adverse events was monitored during the study.
Enzyme-linked immunosorbent assay
All reagents were molecular grade or better and purchased from commercial sources without further purification. Tape strips collected from the study subjects were stored at −80 °C until analyzed. After the tape strips were thawed at room temperature (20 °C), 2 ml of 1% Triton X-100 aqueous solution were added to each tape strip in a sample vial. The samples were agitated for 1 h at room temperature and inverted to ensure consistent mixing. A 1-ml sample of the extraction solution was then centrifuged at 10,000 x g for 2 min, and 50 or 100 µL of the clarified solution was immediately analyzed by enzyme-linked immunosorbent assay (ELISA) to quantify IL-17A or TNF-α levels, respectively, per manufacturer protocol. ELISA kits for TNF-α and IL-17A were purchased from ThermoFisher Scientific (Waltham, MA). IL-17A samples were diluted 2-fold with sample diluent, whereas TNF-α samples were analyzed without further dilution. A VarioSkan Lux UV plate reader (ThermoFisher Scientific) was used to analyze samples, and all analyses were conducted with UV-vis absorbance at 450 nm. TNF-α and IL-17A quantification was validated via standard calibration curves (R2 = 0.9933 and R2 = 0.9962, respectively).
Statistical analysis
A 2-sided t-test was used to analyze paired data for the presence of TNF-α and IL-17A in psoriasis plaques, and a p value ≤0.05 was considered statistically significant.
Results
Baseline characteristics
Ten subjects were enrolled and completed the study (male, n = 5; female, n = 5); however, 1 subject was removed (male, n = 5; female, n = 4) from final analyses because of a protocol violation (self-debridement, which induced koebnerization of treated and untreated plaques). The majority of subjects (7 of 9) were Caucasian, 1 of 9 was Hispanic, and 1 of 9 was African American. A higher proportion of subjects were type II on the Fitzpatrick skin type scale (6 of 9; 66.7%), with 33.3% classified as type I, IV, or V (1 subject in each category).
Investigator’s Global Assessment scores and target lesion Assessment
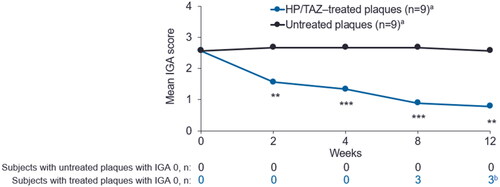
No significant difference in baseline Investigator’s Global Assessment (IGA) score was observed between the treated and untreated plaques. Significant improvement in mean IGA score in HP/TAZ–treated compared to untreated plaques was evident as early as week 2 (p = 0.0027) and maintained through week 12 (p = 0.0012; ).
Figure 1. Mean plaque IGA scores of HP/TAZ–treated and untreated plaques through week 12. HP/TAZ, halobetasol propionate 0.01% and tazarotene 0.045%; IGA, investigator’s Global Assessment. aOne of the 10 enrolled subjects was removed for protocol violation and not included in analyses. bTwo subjects maintained an IGA score of 0 from week 8 to week 12. **p < 0.005, ***p < 0.001, treated compared to untreated plaques (n = 9).
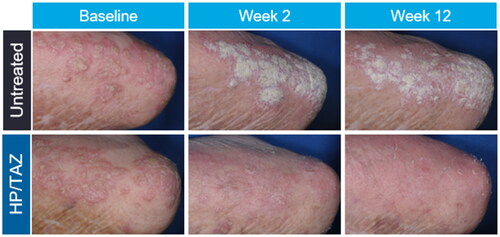
At baseline, there were no significant differences in investigator-assessed lesion characteristics between HP/TAZ–treated and untreated plaques. By week 2, there was a significant improvement in erythema (p = 0.003), induration (p = 0.002), and scaling (p = 0.002) in HP/TAZ–treated compared to untreated plaques. Statistically significant lesion improvement (p ≤ 0.002) continued in all parameters from weeks 4 through 12. Similarly, there were no significant differences in subject-assessed lesion characteristics at baseline between target plaques. By week 2, subject-assessed redness (p < 0.001), thickness (p < 0.001), and scaling (p < 0.001) were significantly reduced in HP/TAZ–treated compared to untreated plaques; furthermore, statistically significant lesion improvement (p ≤ 0.011) continued in all subject-assessed parameters from weeks 4 through 12. Representative images of HP/TAZ–treated compared to untreated target lesions from baseline to week 12 are shown in .
TNF-α concentration in psoriatic plaques
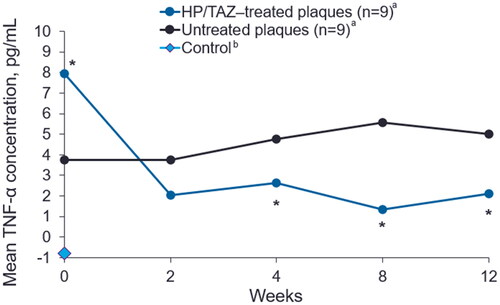
At all post-baseline time points, HP/TAZ treatment was associated with reductions in TNF-α levels in treated plaques compared to untreated plaques (). The baseline concentration of TNF-α was significantly higher in HP/TAZ–treated compared to untreated lesions (7.94 pg/mL vs 3.74 pg/mL, respectively; p = 0.041); however, TNF-α levels were numerically reduced to 2.04 pg/mL after 2 weeks of treatment with HP/TAZ lotion compared to untreated lesions, which showed no reduction at week 2 (3.74 pg/mL; p = 0.228). TNF-α levels were significantly lower in HP/TAZ–treated compared to untreated plaques at week 4 (p = 0.025); this reduction was maintained through week 12 (p = 0.013). TNF-α was not detected above the limit of quantification (0.24 pg/mL) in negative control samples from healthy skin.
Figure 3. Mean TNF-α levels in HP/TAZ–treated and untreated plaques. HP/TAZ, halobetasol propionate 0.01% and tazarotene 0.045%; TNF-α, tumor necrosis factor alpha. aOne of the 10 enrolled subjects was removed for protocol violation and not included in analyses. bControl: D-squame tape was taken from a healthy volunteer without psoriasis. *p < 0.05, treated compared to untreated plaques (n = 9).
IL-17A Concentration in psoriatic plaques
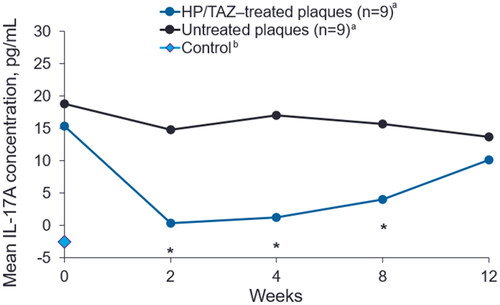
Baseline IL-17A levels were similar between HP/TAZ–treated and untreated plaques (15.37 pg/mL vs 18.81 pg/mL, respectively; p = 0.376; ). From weeks 2 through 8, levels of IL-17A were significantly reduced in HP/TAZ–treated plaques compared to untreated plaques (p < 0.05). At week 12, IL-17A concentrations were numerically lower in HP/TAZ–treated compared to untreated plaques (10.11 pg/mL vs 13.62 pg/mL, respectively; p = 0.142). IL-17A was not detected above the limit of quantification (3.2 pg/mL) in negative control samples.
Figure 4. Mean IL-17A levels in HP/TAZ–treated and untreated plaques. HP/TAZ, halobetasol propionate 0.01% and tazarotene 0.045%; IL-17A, interleukin 17 A. aOne of the 10 enrolled subjects was removed for protocol violation and not included in analyses. bControl: D-squame tape was taken from a healthy volunteer without psoriasis. *p < 0.05, treated compared to untreated plaques (n = 9).
TNF-α and IL-17A Concentration after HP/TAZ discontinuation
Three subjects achieved an IGA score of 0 in treated plaques at week 8 and discontinued HP/TAZ treatment. At 4 weeks posttreatment cessation (week 12), 2 of these 3 subjects had maintained an IGA score of 0; the third subject had an IGA score of 1. In the HP/TAZ–treated plaques of these 3 subjects, TNF-α and IL-17A levels remained low from week 8 to week 12 (e.g., following treatment cessation). Mean TNF-α levels in HP/TAZ–treated plaques of subjects who maintained an IGA score of 0 and discontinued treatment were 0.08 pg/mL at week 8 and 1.18 pg/mL at week 12; mean IL-17A levels were −0.91 pg/mL at week 8 and 0.64 pg/mL at week 12.
Safety
No adverse events were reported during the study.
Discussion
In this study, HP/TAZ was highly effective in treating plaque psoriasis, as demonstrated by the significant improvement in mean investigator IGA scores and by improvements in both investigator- and subject-reported assessments of treated compared to untreated plaques. The efficacy of HP/TAZ in this analysis is corroborated with statistically significant decreases in TNF-α and IL-17A, 2 cytokines implicated in the pathogenesis of psoriasis.
The primary efficacy endpoint was a decrease in TNF-α or IL-17A in HP/TAZ–treated plaques after achieving an IGA score of 0. Although statistically significant reductions in TNF-α and IL-17A levels were observed in treated plaques compared to untreated plaques at most time points, not all subjects achieved an IGA of 0 with HP/TAZ. Three subjects achieved an IGA score of 0 in treated plaques at week 8 and discontinued HP/TAZ therapy. At week 12 (4 weeks post therapy cessation), the formerly treated plaques of these subjects demonstrated a sustained reduction of mean TNF-α and IL-17A levels. The ability of a topical treatment to maintain downregulation of inflammatory cytokines after discontinuation of therapy has not been well established; however, these data suggest that topical HP/TAZ has the potential to maintain reductions in inflammatory cytokines in psoriatic plaques even after cessation of therapy. Additional studies with larger cohorts are needed to confirm these findings.
The secondary efficacy endpoint was the decrease or maintained decrease in TNF-α or IL-17A after HP/TAZ treatment for 4 weeks, which was met for both cytokines. TNF-α was significantly decreased in treated plaques at weeks 4, 8, and 12 compared to untreated plaques. Of note, there was a statistically significant difference in baseline TNF-α levels in treated and untreated plaques; however, the level of TNF-α was higher in treated lesions, which would not bias study results. Additionally, HP/TAZ treatment significantly reduced IL-17A in subject plaques at weeks 2, 4, and 8, with a numerical reduction maintained at week 12. Taken together, HP/TAZ was associated with sustained, substantial reduction in pro-inflammatory cytokines.
There are several potential explanations for how HP/TAZ may facilitate reduction of cytokines associated with psoriasis. Corticosteroids, like HP, act at the cellular level by binding with the glucocorticoid receptor (Citation24) and have various functions, including anti-inflammatory and immunomodulatory actions (Citation17). A potential mechanism for the reduction in TNF-α observed here with HP/TAZ treatment is the ability of HP to modulate TNF-α gene expression via the glucocorticoid receptor (Citation22). Additionally, corticosteroids suppress macrophage secretion of TNF-α and reduce its expression on cell membranes. Further, TAZ is a retinoid prodrug that is rapidly metabolized to tazarotenic acid, which selectively binds to retinoic acid receptors and serves as a ligand-dependent transcription factor to regulate the expression of psoriasis-associated genes, including TNF-α and IL-17 (Citation22,Citation25). TAZ halts a positive inflammatory feedback loop for epidermal inflammation by downregulating migration inhibitory factor-related protein 8 (MRP-8), a protein stimulated by TNF-α that recruits leukocytes to enhance expression of TNF-α and other pro-inflammatory cytokines at inflammatory sites (Citation22). TNF-α also has been shown to stimulate Th17 cells to produce IL-17 in human peripheral blood mononuclear cells (Citation26,Citation27); thus, it could be hypothesized that HP/TAZ reduces IL-17A levels by diminishing TNF-α–induced Th17 stimulation. In sum, HP/TAZ treatment may reduce inflammatory markers in psoriatic lesions through several mechanisms, ultimately normalizing plaque inflammation and returning skin to a ‘prelesional state’ (Citation22); additional studies are needed to clarify this mechanism of action.
The results of this study may also inform the treatment of patients with scalp or palmoplantar psoriasis, which are especially challenging to treat and contribute to poor quality of life and negative self-image (Citation28). In previous open-label studies, HP/TAZ demonstrated efficacy in treating moderate-to-severe palmoplantar psoriasis and moderate-to-severe psoriasis with scalp involvement (Citation29,Citation30). In these studies, the novel formulation of HP/TAZ was proposed to contribute to the favorable efficacy profile observed. The results of this study may provide further depth to this observation, as the ability of HP/TAZ to reduce TNF-α and IL-17A in psoriatic lesions may contribute to its efficacy in difficult-to-treat psoriatic subtypes. To confirm this, further studies of cytokine reduction associated with HP/TAZ are warranted in difficult-to-treat areas of the body.
Although these study findings suggest the potential for a topical psoriasis therapy to reduce inflammatory cytokines in plaques, there are limitations (Citation31). One limitation is the small sample size, which may obscure statistical analysis. Indeed, 1 subject was excluded because of a protocol violation, reducing the already small pool of subjects analyzed. Additionally, only 3 subjects achieved an IGA score of 0 during the study, which limited analysis of sustained cytokine suppression following cessation of therapy. Future studies with larger cohorts are needed to further explore these results. Lastly, future studies should examine the effect of HP/TAZ on additional pro-inflammatory cytokines implicated in psoriasis.
In conclusion, HP/TAZ was efficacious in treating psoriatic plaques, demonstrated by significant improvements in IGA scores and investigator-reported assessments of erythema, induration, and scaling, which were mirrored in subject-reported assessments. Furthermore, HP/TAZ significantly reduced TNF-α and IL-17A levels in psoriatic plaques, corroborating its efficacy, and represents an advancement in topical therapy and cytokine reduction for patients with psoriasis.
Author’s contributions
ZD received funding from Ortho Dermatologics to conduct the research detailed in this manuscript. MD has no disclosures to report. TL and AJ are employees of Ortho Dermatologics (a division of Bausch Health US, LLC).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available upon reasonable request of the corresponding author.
Additional information
Funding
References
- Grän F, Kerstan A, Serfling E, et al. Current developments in the immunology of psoriasis. Yale J Biol Med. 2020;93:1–6.
- von Stebut E, Boehncke WH, Ghoreschi K, et al. IL-17A in psoriasis and beyond: cardiovascular and metabolic implications. Front Immunol. 2019;10:3096. doi: 10.3389/fimmu.2019.03096.
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2–dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244.
- Chima M, Lebwohl M. TNF inhibitors for psoriasis. Semin Cutan Med Surg. 2018;37(3):134–142. doi: 10.12788/j.sder.2018.039.
- Yost J, Gudjonsson JE. The role of TNF inhibitors in psoriasis therapy: new implications for associated comorbidities. F1000 Med Rep. 2009;1:30. doi: 10.3410/M1-30.
- Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–390. doi: 10.1007/s12016-018-8702-3.
- Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine. 2015;73(2):342–350. doi: 10.1016/j.cyto.2014.12.014.
- Kutwin M, Migdalska-Sęk M, Brzeziańska-Lasota E, et al. An analysis of IL-10, IL-17A, IL-17RA, IL-23A and IL-23R expression and their correlation with clinical course in patients with psoriasis. Journal of Clinical Medicine. 2021;10:5834. doi: 10.3390/jcm10245834.
- Mohd Noor AA, Azlan M, Mohd Redzwan N. Orchestrated cytokines mediated by biologics in psoriasis and its mechanisms of action. Biomedicines. 2022;10:498. doi: 10.3390/biomedicines10020498.
- Immunex Corporation. Enbrel [package insert]. Thousand Oaks (CA): Immunex Corporation; 2022.
- Janssen Biotech I. Remicade [package insert]. Horsham (PA): Janssen Biotech I; 2021.
- AbbVie Inc. Humira [package insert]. North Chicago (IL): AbbVie Inc; 2021.
- Novartis Pharmaceuticals Corporation. Cosentyx [package insert]. East Hanover (NJ): Novartis Pharmaceuticals Corporation; 2021.
- Eli Lilly and Company. Taltz [package insert]. Indianapolis (IN): Eli Lilly and Company; 2016.
- Bausch Health US L. Siliq [package insert]. Bridgewater (NJ): Bausch Health US L; 2017.
- Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics. Nat Rev Drug Discov. 2019;18(1):19–40. doi: 10.1038/nrd.2018.183.
- Uva L, Miguel D, Pinheiro C, et al. Mechanisms of action of topical corticosteroids in psoriasis. Int J Endocrinol. 2012;2012:561018. doi: 10.1155/2012/561018.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD–NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi: 10.1016/j.jaad.2020.07.087.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-Severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855–861.
- Tanghetti EA, Werschler WP, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19(1):70–77. doi: 10.36849/JDD.2020.3977.
- Lebwohl MG, Stein Gold L, Del Rosso JQ, et al. Posttreatment maintenance of therapeutic effect with fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion for moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2022;33(4):2068–2074. doi: 10.1080/09546634.2021.1914310.
- Lebwohl MG, Tanghetti EA, Stein Gold L, et al. Fixed-combination halobetasol propionate and tazarotene in the treatment of psoriasis: narrative review of mechanisms of action and therapeutic benefits. Dermatol Ther (Heidelb). 2021;11(4):1157–1174. doi: 10.1007/s13555-021-00560-6.
- Lebwohl MG, Stein Gold L, Papp K, et al. Long-term safety and efficacy of a fixed-combination halobetasol propionate 0.01%/tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis: phase 3 open-label study. J Eur Acad Dermatol Venereol. 2021;35(5):1152–1160. doi: 10.1111/jdv.17113.
- Ramamoorthy S, Cidlowski JA. Corticosteroids. Rheum Dis Clin North Am. 2016;42(1):15–31, vii. doi: 10.1016/j.rdc.2015.08.002.
- Issa N, Kircik L. Supplement individual article: a reappraisal of fixed-combination halobetasol propionate and tazarotene for the treatment of psoriasis: biological underpinnings, therapeutic mechanisms, and economic considerations. J Drugs Dermatol. 2023;22(1):3446174–34461710.
- Pesce B, Ribeiro CH, Larrondo M, et al. TNF-α affects signature cytokines of Th1 and Th17 T cell subsets through differential actions on TNFR1 and TNFR2. Int J Mol Sci. 2022;23:9306.
- Zheng Y, Sun L, Jiang T, et al. TNFαPromotes Th17 cell differentiation through IL-6 and IL-1βProduced by monocytes in rheumatoid arthritis. J Immunol Res. 2014;2014:385352. doi: 10.1155/2014/385352.
- Kircik L, Tanghetti EA, Friedman A, et al. Challenges in psoriatic disease addressed by Fixed-Combination halobetasol propionate 0.01% and tazarotene 0.045% lotion. J Clin Aesthet Dermatol. 2023;16:21–26.
- Ozyurekoglu E, Kircik L. An open-label pilot study to investigate safety and efficacy of fixed combination tazarotene 0.045% and halobetasol propionate 0.01% lotion for the treatment of scalp psoriasis. J Drugs Dermatol. 2021;20(11):1191–1194. doi: 10.36849/jdd.0102.
- Campbell CR, Babalola FO, Yousif JE. 33385 A polymeric emulsion of halobetasol propionate and tazarotene in the treatment of palmoplantar psoriasis. Journal of the American Academy of Dermatology. 2022;87(3):AB10. doi: 10.1016/j.jaad.2022.06.075.
- Steer JH, Kroeger KM, Abraham LJ, et al. Glucocorticoids suppress tumor necrosis factor-α expression by human monocytic THP-1 cells by suppressing transactivation through adjacent NF-κB and c-Jun-Activating transcription factor-2 binding sites in the promoter. J Biol Chem. 2000;275(24):18432–18440. doi: 10.1074/jbc.M906304199.