Abstract
Mesotherapy is a technique by which lower doses of therapeutic agents and bioactive substances are administered by intradermal injections to the skin. Through intradermal injections, mesotherapy can increase the residence time of therapeutic agents in the affected area, thus allowing for the use of lower doses and longer intervals between sessions which may in turn improve the treatment outcome and patient compliance. This systematic review aims to summarize the current literature that evaluates the efficacy of this technique for the treatment of hair loss and provides an overview of the results observed. Of the 416 records identified, 27 articles met the inclusion criteria. To date, mesotherapy using 6 classes of agents and their combinations have been studied; this includes dutasteride, minoxidil, growth factors or autologous suspension, botulinum toxin A, stem cells, and mesh solutions/multivitamins. While several studies report statistically significant improvements in hair growth after treatment, there is currently a lack of standardized regimens. The emergence of adverse effects after mesotherapy has been reported. Further large-scale and controlled clinical trials are warranted to evaluate the utility of mesotherapy for hair loss disorders.
1. Introduction
Mesotherapy or local intradermal therapy is a minimally invasive method that involves the injection of therapeutic agents such as drugs and bioactive substances into the skin at a depth of 2–4 mm (Citation1). Intradermal injections have been used to treat localized pain, and are also being widely employed in cosmetic dermatology to promote skin rejuvenation, treat pigmentary disorders, and promote hair growth (Citation2,Citation3).
One of the primary advantages of mesotherapy is that the therapeutic agent is administered directly into the skin, bypassing the barriers that would be faced by topical agents, thereby enabling a targeted approach to treatment (Citation4). As a result, it is hypothesized that the bioavailability of the agent is increased due to longer residence time at the injected site, in addition to its direct presence in the targeted area (Citation1,Citation4). Studies have reported that lower doses of drugs can be used with longer time intervals between sessions which could lead to improved patient compliance (Citation5). Additionally, as the therapeutic agent remains near the injected area, the potential for systemic spread to other areas of the body as observed with oral treatment is lower, which may lead to decreased off-target adverse effects (Citation3).
While the term “mesotherapy” was coined in the latter half of the twentieth century, the utility of this treatment for hair loss was not investigated until recently (Citation2,Citation3). Selecting the optimal treatment that can induce significant and durable hair regrowth remains challenging, despite the availability of several agents approved by regulatory bodies around the world (Citation6–9). Studies have shown that the administration of lower doses of approved and emerging agents through mesotherapy, directly to the scalp, may be an effective treatment for hair loss (Citation4). In this systematic review, we aim to provide a summary of the published trials and reports that have used mesotherapy to treat hair loss.
2. Methods
An electronic search was conducted to gather studies on the use of mesotherapy to treat hair loss. The systematic review was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline in March 2023 using PubMed, Embase (Ovid), Scopus, and Web of Science, without date restrictions (Citation10). The search terms used were “mesotherapy” and “hair loss”. The deduplication, and screening of titles and abstracts, were performed using Rayyan (https://www.rayyan.ai/). Articles were excluded if they were reviews or commentaries, did not contain human data, were not treating hair loss, did not use mesotherapy, or if they were not available in English. Articles included after the title and abstract screening were retrieved and assessed for eligibility. The reference sections of relevant review articles were screened for any additional eligible studies. Studies used for data extraction in this unregistered systematic review involved the use of mesotherapy to administer drugs/therapeutic agents as monotherapy or in combination with other agents and described therapeutic or adverse effects in patients. The quality of the studies was assessed using the 2011 Oxford Center of Evidence-Based Medicines levels of evidence. The collection of data was performed using Excel (Citation11).
3. Results and discussion
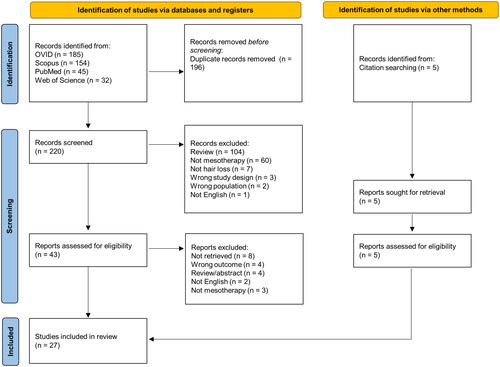
A total of 416 records were obtained from the literature search, of which 27 were found to be eligible and included in the systematic review (). Of the 27 included studies, 19 studies report on the regrowth of hair using mesotherapy ( and ) and 8 studies (case series and case reports, ) discuss the occurrence of adverse effects due to mesotherapy. Among the 19 studies reporting on hair regrowth, 10 were randomized controlled trials, 1 was a non-randomized controlled trial, 4 were prospective cohort or observational studies, 2 were retrospective studies, and 2 were case reports. Pattern hair loss or androgenetic alopecia (AGA) was found to be the primary hair loss disorder treated using mesotherapy (18/19) in the studies discussing hair regrowth, while telogen effluvium (TE) was less common (1/19). The geographical locations of the study sites are summarized in . A number of agents were administered via mesotherapy to treat hair loss. In the following subsections, these agents, their regimens, efficacies and safety will be discussed.
Figure 2. Global reports of studies on mesotherapy for hair loss. The greatest number of studies on mesotherapy so far have been reported from Egypt, followed by India, Spain, and Brazil. A total of 10 clinical trials were identified, in addition to observational studies, case series, and reports. Image created with MapChart.
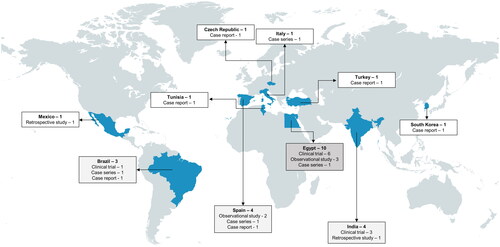
Table 1. Summary of randomized controlled trials on mesotherapy for hair loss.
Table 2. Summary of non-randomized controlled trials, studies, and reports on mesotherapy in patients with pattern hair loss.
Table 3. Summary of studies and reports on adverse effects associated with mesotherapy.
3.1. Agents used for mesotherapy
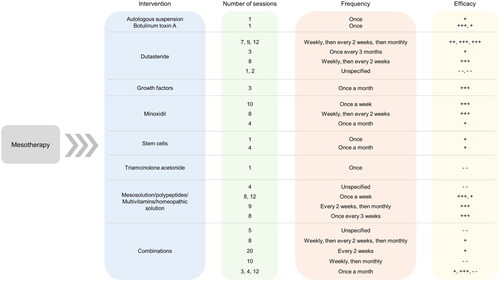
Various therapeutic agents, ranging from dutasteride and minoxidil to stem cells, have been administered via intradermal injections to the scalp (). In some studies, a combination of these agents was found to be more effective due to their synergistic effects. The following agents have been explored so far to treat PHL/AGA and TE using mesotherapy.
Figure 3. Summary of Interventions, regimens, and the Efficacies of agents used for mesotherapy to treat hair loss. Data from 26 of the 28 studies that reported the number and frequency of sessions for each intervention have been summarized. Here, “+++” refers to statistically significant improvement in trichoscopic parameters (total or terminal hair count, total or terminal hair density, hair diameter, vellus hair count or density) or hair regrowth percentage, “++” refers to statistically significant improvement in global photographic/physician assessment or patient self-assessment, “+” refers to improvement in any of the parameters without statistical significance, “–” refers to no improvement in any of the parameters, and “– –” refers to adverse effects observed in response to mesotherapy.
3.2. Dutasteride
Dutasteride is an inhibitor of the 5-α-reductase enzyme that inhibits the conversion of free testosterone to dihydrotestosterone, which is hypothesized to be the major contributor to AGA pathogenesis (Citation12,Citation13). When administered orally, a range of doses of dutasteride (0.05 − 2.5 mg daily) has been evaluated, with 0.5 mg daily being the most commonly reported and commercially available dosage (Citation12). Using mesotherapy, the range of concentrations of dutasteride evaluated was 0.005 − 0.05%, administered at weekly, bi-monthly, or monthly intervals to treat PHL ( and ) (Citation14–16). Mesotherapy reduces the frequency and dosage of administration, leading to the potential reduction of systemic off-target adverse effects (Citation12).
3.3. Minoxidil
Minoxidil is a vasodilator that is FDA-approved for the treatment of PHL, and is involved in the prolongation of the anagen phase of hair follicles leading to increased hair growth (Citation17). A topical solution of minoxidil 2% and a minoxidil 5% foam applied twice daily have been FDA-approved, while concentrations of 1% to 5% have been investigated for the treatment of other hair loss disorders (Citation18). For PHL, mesotherapy with minoxidil 0.5% and 2% solutions administered at weekly or bi-monthly intervals were investigated () (Citation19,Citation20). It is hypothesized that the injection of minoxidil may increase its therapeutic effect and lead to higher patient compliance (Citation19,Citation20).
3.4. Growth factors and autologous suspensions
Plasma and autologous suspensions rich in growth factors such as fibroblast growth factor, vascular endothelial growth factor, transforming growth factor β1 and epidermal growth factor have been hypothesized to induce the regeneration and subsequent regrowth of hair follicles (Citation21,Citation22). Two studies used mesotherapy to administer lyophilized growth factors or an autologous suspension of cells with growth factors to treat PHL; however, the constituents and concentrations were not specified () (Citation23,Citation24). Additional studies using standardized doses are required to better analyze the utility and safety of these agents (Citation4).
3.5. Botulinum toxin A
Botulinum toxin A is widely used in cosmetic dermatology and has recently been explored to treat hair loss (Citation4,Citation25). It is typically administered via injections and due to its vasodilatory effect, it is hypothesized to relax the scalp and thereby increase blood flow and oxygenation, resulting in the reduction of hair loss and regrowth of hair (Citation26). For PHL, 50 units of botulinum toxin A were injected into the scalp in one session; while 100 units were used for TE () (Citation26,Citation27).
3.6. Stem cells
Stem cells, being pluripotent or multipotent depending on their source, are able to regenerate and differentiate into a variety of cell types. As a result, stem cells are being explored to treat PHL due to their potential to differentiate into dermal papilla cells leading to hair follicle regeneration and hair regrowth (Citation1,Citation28,Citation29). In two studies included in this review, adipose-derived stem cells and neonatal-derived mesenchymal stem cells were administered once and at monthly intervals, respectively () (Citation30,Citation31). Details pertaining to the suspension administered were not provided. Further large-scale studies and detailed reports on the suspensions used are required to standardize this treatment option.
3.7. Mesosolutions/polypeptides/multivitamins/homeopathic solution
Other than the conventional or upcoming agents, there are solutions consisting of amino acids, peptides, vitamins, minerals, plant extracts/growth factors, and homoeopathic components being explored to treat hair loss (Citation26,Citation27,Citation32–35). While the rationale for administering these agents using mesotherapy has been described as the ability to increase blood flow and stimulate the growth of hair, the mechanism of action in achieving this effect has not been described to date (Citation1). In all the studies included in this review that used these solutions, detailed reports of the constituents of these preparations have not been provided, which again emphasizes the need to elaborate on the therapeutic effects and the safety of these components in order to develop standardized protocols of mesotherapy for hair loss.
3.8. Combinations
Of the 19 studies reporting on hair regrowth listed in and , five studies involved the use of combination treatments. Dutasteride was combined with biotin, pyridoxin and D-panthenol or oral minoxidil in four studies (Citation13–15,Citation36). In another study, minoxidil was combined with finasteride, biotin, and D-panthenol (Citation37). The synergistic effect of these agents led to the improvement in hair growth in a majority of the cases, indicating their potential in addressing hair loss (Citation1).
3.9. Regimens
The regimens reported for mesotherapy in the 27 studies included in this systematic review are diverse, owing to the multiple agents used. The number of sessions ranges from 1 session (for stem cells, botulinum toxin A, and autologous suspension) to 20 sessions (for a solution containing minoxidil, finasteride, biotin, and D-panthenol) (). However, there is an absence of consistency even when the same agent is used; for example, the number of sessions reported for dutasteride mesotherapy ranges from 1 to 12 (). The frequency of the injections ranges from once a week to once every 3 months. Most injections are performed once a week or once a month, while many begin once a week, and then progress to once in 2 weeks and/or once a month (Citation13,Citation15,Citation26,Citation32). This indicates that as the treatment progresses, the frequency of the injections may be decreased, which could lead to higher patient compliance (Citation13).
3.10. Techniques
Mesotherapy is typically performed using 28–30 gauge needles or with a mesogun in sessions lasting between 10–30 min (Citation20,Citation38). To reduce musculoskeletal strain caused by performing multiple injections in one sitting, the mesogun can be utilized in some instances to deliver consistent and faster injections (Citation38). There are five techniques that are commonly used and they vary in needle depth and/or angle of injection. The (1) intra-epidermal or epidermic technique (injection depth: 1 mm) injects multiple small quantities of the mesotherapy solution, often in a grid-like pattern 1-cm apart, into the epidermis covering the entire treatment area; each injection is applied with a light pressure causing minimal pain and without bleeding, which makes this technique suitable for patients with a low threshold of pain tolerance (Citation38–40), (2) papular or papule-forming technique (injection depth: 2–4 mm) injects the mesotherapy solution at the dermoepidermal junction which causes a small papule to form, and is recommended for the treatment of alopecia or wrinkles (Citation39), the (3) nappage technique (injection depth: 2–4 mm) involves multiple superficial injections performed at a 30–60° angle, with a constant pressure applied to each injection that introduces one drop of mesotherapy solution per site; this technique is widely used for scalp treatments and skin rejuvenation, however it could cause more patient discomfort compared to other techniques (Citation1,Citation38,Citation39), (4) point-by-point technique (injection depth: 4 mm) involves perpendicular deep injections of 0.02–0.05 ml mesotherapy solutions, 1–2 cm apart, into the dermis (Citation24,Citation38,Citation39), and (5) mesoperfusion technique that involves injections of the same mesotherapy solution over a 10-minute period (Citation40). The napping and point-by-point techniques are most commonly used for the treatment of hair loss by mesotherapy (Citation13,Citation14,Citation30,Citation37).
To minimize patient discomfort, several alternative needleless procedures have been described that can potentially prevent skin reactions while maintaining a similar level of drug penetration (Citation39,Citation40). This includes the application of low-power ultrasound (20-25 KHz) that may enhance skin permeability through the formation of small gas cavities, and iontophoresis that facilitates transdermal drug delivery through the application of an electric current (≤0.5 mA/cm2) (Citation41).
3.11. Efficacy
Due to the diversity of therapeutic agents administered through mesotherapy for the treatment of hair loss, we observed varying degrees of efficacy reported in the literature. Among the 10 RCTs, 7 studies reported statistically significant improvements in more than one assessment criteria: trichoscopic parameters (total or terminal hair density or hair count, hair diameter, terminal to vellus hair ratio, etc.), physician assessment, or patient assessment compared to baseline or control (). The agents evaluated were minoxidil, dutasteride, botulinum toxin A, multivitamins, and a plant-derived polypeptide solution. While botulinum toxin A is effective as monotherapy, other agents show improved efficacies when used in combination with each other or with the addition of vitamins and amino acids (Citation1). For example, Sobhy et al. showed that a solution of dutasteride containing biotin, pyridoxine and D-panthenol resulted in greater improvements in 30 patients in terms of the hair shaft diameter, compared to patients who received dutasteride monotherapy (Citation15). The combined effect of these agents appears to result in improved therapeutic effects; however, the reasons for these observations and their mode of action have not been further investigated.
Of the 19 studies showing hair regrowth, 9 studies were not RCTs. Statistically significant improvements in more than one assessment criteria were reported in 3 studies that evaluated the efficacy of dutasteride and lyophilized growth factors (Citation14,Citation24,Citation42). Improvement, with no confirmation of statistical significance, was seen in 5 studies while no improvement was reported in 1 study (). Newer agents like stem cells, and combinations such as minoxidil and finasteride, were evaluated in smaller-sized patient cohorts or in single patients indicating the need for further analysis to confirm their therapeutic effect when administered using mesotherapy.
No conclusions or correlations could be made between the regimens used and the efficacies observed due to the high degree of variability between studies. In the case of AGA, it was found that mesotherapy was more effective in patients presenting with a shorter duration of prior hair loss due to a lesser degree of damage to the hair follicles (Citation13,Citation14). Mesotherapy using minoxidil, or botulinum toxin A with multivitamins, was found to be effective leading to significant hair regrowth in TE patients (Citation27). However, further RCTs are required to confirm the utility of this treatment for TE.
3.12. Safety and adverse effects
The most common side effects of mesotherapy reported in the RCTs, observational studies, and prospective studies were mild and resolved in a few days (Citation1). Headache, injection site pain, and scalp tightness or itching were most frequently reported, while some studies also mentioned redness and swelling ( and ). Nine of the 19 studies that observed hair regrowth did not report any adverse effects.
On the other hand, eight of the 27 studies included in this systematic review reported adverse effects of mesotherapy (). All of these studies are case series or case reports, with mesotherapy administered to patients with PHL, TE, or severe migraine. The reported adverse effects include hair loss at the injection site, edema, swelling, scalp melanoma, and frontal fibrosing alopecia (). These side effects were reported after sessions of mesotherapy injections with agents such as growth factor solution, dutasteride solution, mesoglycan, homeopathic solution, triamcinolone acetonide, or propranolol. Associations between these agents or mesotherapy with the adverse effects are unclear; however, patients with a known history of hypersensitivity reactions to ingredients, congenital nevus or prior surgical trauma near the injection site, may be predisposed to a higher risk (Citation43–45). Additional risk factors include drug-drug interactions, and possible product contaminations (Citation1,Citation5). Further investigation and deeper analyses are required to elaborate on these potential outcomes of mesotherapy (Citation1).
4. Conclusions
Mesotherapy is a method of intradermal administration of drugs and bioactive substances with the potential of treating hair loss disorders such as PHL and TE. Several studies have shown statistically significant improvements in hair growth after mesotherapy injections utilizing various therapeutic agents and homoeopathic solutions. The use of lower drug doses, localized administration, and lower frequency of injections are factors that can improve the utility of mesotherapy. However, mesotherapy is currently not approved by the U.S. FDA or Health Canada, and given the reports of serious adverse effects observed after mesotherapy sessions with some agents, it is advised to proceed with caution when using this empirical treatment. To reduce the possible development of allergic reactions to the therapeutic agents or the preparations being injected, pretreatment allergy testing should be considered when appropriate and clinically indicated. Currently, no standardized treatment regimen exists for mesotherapy, which necessitates further large-scale, controlled clinical trials to optimize its efficacy and safety profile.
Author contributions
Conceptualization, A.K.G.; Data Curation, S.P.R.; Formal Analysis, S.P.R.; Investigation, S.P.R.; Methodology, S.P.R. and T.W.; Project Administration, A.K.G.; Supervision, A.K.G.; Validation, A.K.G., T.W., M.T., M.S. and B.M.P.; Visualization, S.P.R.; Writing – Original Draft Preparation, S.P.R.; Writing – Review and Editing, A.K.G., T.W., M.T., M.S. and B.M.P.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Tang Z, Hu Y, Wang J, et al. Current application of mesotherapy in pattern hair loss_ a systematic review. J Cosmet Dermatol. 2022;21(10):1–11. doi: 10.1111/jocd.14900.
- Mysore V. Mesotherapy in management of hairloss – is it of any use. Int J Trichology. 2010;2(1):45–46. doi: 10.4103/0974-7753.66914.
- Mammucari M, Maggiori E, Russo D, et al. Mesotherapy: from historical notes to scientific evidence and future prospects. Sci World J. 2020;2020:3542848.
- Müller Ramos P, Melo DF, Radwanski H, et al. Female pattern hair loss: therapeutic update. An Bras Dermatol. 2023;S0365-0596:00053–00053.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48(8):855–861. doi: 10.1097/DSS.0000000000003498.
- Chien Yin GO, Siong-See JL, Wang ECE. Telogen effluvium – a review of the science and current obstacles. J Dermatol Sci. 2021;101(3):156–163. doi: 10.1016/j.jdermsci.2021.01.007.
- Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76(14):1349–1364. doi: 10.1007/s40265-016-0629-5.
- Ramírez-Marín HA, Tosti A. Emerging drugs for the treatment of alopecia areata. Expert Opin Emerg Drugs. 2022;27(4):379–387. doi: 10.1080/14728214.2022.2149735.
- Gupta AK, Wang T, Polla Ravi S, et al. Systematic review of newer agents for the management of alopecia areata in adults: Janus kinase inhibitors, biologics and phosphodiesterase-4 inhibitors. J Eur Acad Dermatol Venereol. 2023;37(4):666–679. doi: 10.1111/jdv.18810.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence [Internet]. 2011 [cited 2023 May 24]. Available from: http://www.cebm.net/index.aspx?o=5653.
- Herz-Ruelas ME, Álvarez-Villalobos NA, Millán-Alanís JM, et al. Efficacy of intralesional and oral dutasteride in the treatment of androgenetic alopecia: a systematic review. Skin Appendage Disord. 2020;6(6):338–345. doi: 10.1159/000510697.
- Abdallah M, El-Zawahry K, Besar H. Mesotherapy using dutasteride-containing solution in male pattern hair loss: a controlled pilot study. J Pan-Arab League Dermat. 2009;20:137–145.
- Moftah N, Moftah N, Abd-Elaziz G, et al. Mesotherapy using dutasteride-containing preparation in treatment of female pattern hair loss: photographic, morphometric and ultrustructural evaluation. J Eur Acad Dermatol Venereol. 2013;27(6):686–693. doi: 10.1111/j.1468-3083.2012.04535.x.
- Sobhy N, Aly H, El Shafee A, et al. Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males. Our Dermatol Online. 2013;4(1):40–45. doi: 10.7241/ourd.20131.08.
- Saceda-Corralo D, Rodrigues-Barata A, Vano-Galvan S, et al. Mesotherapy with dutasteride in the treatment of androgenetic alopecia. Int J Trichology. 2017;9(3):143–145. doi: 10.4103/ijt.ijt_73_16.
- Do Nascimento IJB, Harries M, Rocha VB, et al. Effect of oral minoxidil for alopecia: systematic review. Int J Trichology. 2020;12(4):147–155. doi: 10.4103/ijt.ijt_19_20.
- Sung CT, Juhasz MLW, Choi FD, et al. The efficacy of topical minoxidil for non-scarring alopecia: a systematic review. J Drugs Dermatol. 2019;18:155–160.
- Uzel BPC, Takano GHS, Chartuni JCN, et al. Intradermal injections with 0.5% minoxidil for the treatment of female androgenetic alopecia: a randomized, placebo-controlled trial. Dermatol Ther. 2021;34:e14622.
- Azam MH, Morsi HM. Comparative study between 2% minoxidil topical spray vs. Intradermal injection (mesotherapy) for treatment of androgenetic alopecia in female patients: a controlled, 4-month randomized trial. Egyptian Dermatol Online J. 2010;6:5.
- Anitua E, Pino A, Martinez N, et al. The effect of plasma rich in growth factors on pattern hair loss: a pilot study. Dermatol Surg. 2017;43(5):658–670. doi: 10.1097/DSS.0000000000001049.
- Kieb M, Sander F, Prinz C, et al. Platelet-Rich plasma powder: a new preparation method for the standardization of growth factor concentrations. Am J Sports Med. 2017;45(4):954–960. doi: 10.1177/0363546516674475.
- Álvarez X, Valenzuela M, Tuffet J, et al. Microscopic and histologic evaluation of the regenera® method for the treatment of androgenetic alopecia in a small number of cases. Int J Res Med Health Sci. 2017;2:19–22.
- El Samahy MH, Fahmy HM, El Sawaf SI, et al. Lyophilized growth factor intralesional injection in female pattern hair loss: a clinical and trichoscopic study. Dermatol Ther. 2021;34:e14867.
- Carloni R, Pechevy L, Postel F, et al. Is there a therapeutic effect of botulinum toxin on scalp alopecia? Physiopathology and reported cases: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2020;73(12):2210–2216. doi: 10.1016/j.bjps.2020.05.035.
- Nassar A, Abdel-Aleem H, Samir M, et al. Efficacy of botulinum toxin a injection in the treatment of androgenic alopecia: a comparative controlled study. J Cosmet Dermatol. 2022;21(10):4261–4268. doi: 10.1111/jocd.14817.
- Khattab FM, Rady A, Khashaba SA. Recent modalities in treatment of telogen effluvium: comparative study. Dermatol Ther. 2022;35:e15720.
- Krefft-Trzciniecka K, Piętowska Z, Nowicka D, et al. Human stem cell use in androgenetic alopecia: a systematic review. Cells. 2023;12:951. doi: 10.3390/cells12060951.
- Gentile P, Garcovich S. Systematic review of platelet-rich plasma use in androgenetic alopecia compared with minoxidil®, finasteride®, and adult stem cell-based therapy. Int J Mol Sci. 2020;21:2702. doi: 10.3390/ijms21082702.
- Budamakuntla L, Loganathan E, Suryanarayana S, et al. Neonatal derived mesenchymal stem cell mesotherapy in androgenetic alopecia: a retrospective observational study and review of literature. Int J Sci Rep. 2015;1(1):32–35. doi: 10.18203/issn.2454-2156.IntJSciRep20150197.
- Coban YK. A preliminary result of adipose derived stem cell treatment for female pattern hair loss. J Cosmet Dermatol. 2022;21(3):1303–1304. doi: 10.1111/jocd.14183.
- Gajjar P, Mehta H, Barvaliya M, et al. Comparative study between mesotherapy and topical 5% minoxidil by dermoscopic evaluation for androgenic alopecia in male: a randomized controlled trial. Int J Trichology. 2019;11(2):58–67. doi: 10.4103/ijt.ijt_89_18.
- Hunter N, Sayed K, Hay RA, et al. Comparing the efficacy of mesotherapy to topical minoxidil in the treatment of female pattern hair loss using ultrasound biomicroscopy: a randomized controlled trial. Acta Dermatovenerol Croat. 2019;27:1–7.
- Shome D, Kapoor R, Doshi K, et al. Comparison of QR 678® & QR678® neo as monotherapy and as combination therapy with 5% minoxidil solution and oral finasteride in the treatment of male androgenetic alopecia—which is better? J Cosmet Dermatol. 2021;20(6):1763–1765. doi: 10.1111/jocd.14154.
- Shome D, Kapoor R, Vadera S, et al. Evaluation of efficacy of intradermal injection therapy vs derma roller application for administration of QR678 neo® hair regrowth formulation for the treatment of androgenetic alopecia—a prospective study. J Cosmet Dermatol. 2021;20(10):3299–3307. doi: 10.1111/jocd.14139.
- Villarreal-Villarreal CD, Boland-Rodriguez E, Rodríguez-León S, et al. Dutasteride intralesional microinjections in combination with oral minoxidil vs. oral minoxidil monotherapy in men with androgenetic alopecia: a retrospective analysis of 105 patients. J Eur Acad Dermatol Venereol. 2022;36(7):e570–e572. doi: 10.1111/jdv.18066.
- Melo DF, de Mattos Barreto T, Plata GT, et al. Excellent response to mesotherapy as adjunctive treatment in male androgenetic alopecia. J Cosmet Dermatol. 2020;19(1):75–77. doi: 10.1111/jocd.12983.
- Sivagnanam G. Mesotherapy – the french connection. J Pharmacol Pharmacother. 2010;1(1):4–8. doi: 10.4103/0976-500X.64529.
- Vedamurthy M. Mesotherapy. Indian J Dermatol Venereol Leprol. 2007;73(1):60–62. doi: 10.4103/0378-6323.30661.
- Konda D, Thappa DM. Mesotherapy: what is new? Indian J Dermatol Venereol Leprol. 2013;79(1):127–134. doi: 10.4103/0378-6323.104689.
- Alsalhi W, Alalola A, Randolph M, et al. Novel drug delivery approaches for the management of hair loss. Expert Opin Drug Deliv. 2020;17(3):287–295. doi: 10.1080/17425247.2020.1723543.
- Moftah N, Mubarak R, Abdelghani R. Clinical, trichoscopic, and folliscopic identification of the impact of metabolic syndrome on the response to intradermal dutasteride 0.02% injection in patients with female pattern hair loss: a prospective cohort study. J Dermatolog Treat. 2021;32(7):827–836. doi: 10.1080/09546634.2019.1708849.
- In Lee Y, Lee M, Lee S, et al. A permanent hair loss in a patient with hypersensitivity to intralesional triamcinolone injection. Ann Dermatol. 2019;31(2):217–220. doi: 10.5021/ad.2019.31.2.217.
- Arenbergerova M, Arenberger P, Gkalpakiotis S, et al. Scalp melanoma after antihair loss mesotherapy. J Eur Acad Dermatol Venereol. 2018;32(5):e187–e188. doi: 10.1111/jdv.14690.
- Frioui R, Mokni S, Tabka M, et al. Frontal fibrosing alopecia following beta-blocker injectable mesotherapy: is it more than a simple coincidence? Dermatol Ther. 2022;35:e15206.
- Melo DF, Saceda-Corralo D, Tosti A, et al. Frontal edema due to mesotherapy for androgenetic alopecia: a case series. Dermatol Ther. 2022;35:e15247.
- EL-Komy M, Hassan A, Tawdy A, et al. Hair loss at injection sites of mesotherapy for alopecia. J Cosmet Dermatol. 2017;16(4):e28–e30. doi: 10.1111/jocd.12320.
- Duque-Estrada B, Vincenzi C, Misciali C, et al. Alopecia secondary to mesotherapy. J Am Acad Dermatol. 2009;61(4):707–709. doi: 10.1016/j.jaad.2008.11.896.
- Reguero Del Cura L, De Quintana Sancho A, Rubio LM, et al. Two cases of paradoxical nonscarring alopecia after mesotherapy with dutasteride. Skin Appendage Disord. 2022;8(1):46–48. doi: 10.1159/000518043.
- Magdaleno-Tapial J, Valenzuela-Oñate C, García-Legaz-Martínez M, et al. Angioedema-like contact dermatitis caused by mesotherapy with dutasteride. Contact Dermatitis. 2020;83(3):246–247. doi: 10.1111/cod.13585.