Dear Editor,
Atopic dermatitis (AD) is a widespread inflammatory skin condition affecting individuals of all age groups. Recent research has identified AD in older patients as a distinct clinical entity (Citation1). Not only, when AD starts after 20 years of age (adult-onset AD), it has been found to have different skin and blood molecular profiles compared to childhood-onset AD, suggesting systemic inflammation beyond Th2-skewing (Citation2). However, treatment in these patients presents a significant challenge due to the higher risk of comorbidities and side effects of traditional systemic medications like cyclosporin (Citation3). Moreover, information regarding the effectiveness and safety of emerging therapies such as Janus kinase (JAK) inhibitors is still limited (Citation4,Citation5).
Upadacitinib, a selective JAK1 inhibitor, has received approval for treating moderate-to-severe AD in adolescents and adults. Nevertheless, information about its efficacy and safety in elderly individuals remains limited due to the inclusion criteria of the registration studies (Citation6,Citation7).
We report a case series of seven elderly patients with adult-onset moderate-to-severe AD receiving upadacitinib. Their demographic and clinical characteristics are summarized in . The choice of the starting dose was done before the publication of the European Medicine Agency (EMA) recommendations to settle the recommended dose for the elderly population as 15 mg daily (Citation8).
Table 1. Demographic and clinical characteristics of patients.
Patient one, a 75-year-old woman, had a medical history that included ovarian carcinoma with pulmonary metastases that was treated with chemotherapy in 2009 and had been in remission since then. She also had pulmonary sarcoidosis (in remission at the time of our evaluation) and two episodes of venous cerebral thrombosis. After being diagnosed with atopic dermatitis with a eczema prurigo nodularis-like phenotype, she was started on a 30 mg/day upadacitinib regimen, which was halved after eight weeks due to good clinical response and disease stabilization. A follow-up visit at 104 weeks showed significant improvement in her Eczema Area and Severity Index (EASI) score.
Patients two, three, and four, aged 65, 68, and 75 years old respectively, started with a daily dose of 30 mg of upadacitinib. Following disease stabilization, their dosages were halved after 52, 24, and 16 weeks, respectively. The patients maintained a complete remission at week 72nd, 104th, and 32nd weeks respectively. Patient four was affected by diabetes and had a foot skin infection which was effectively treated with systemic antibiotic therapy. He also experienced mild anemia with a hemoglobin value of 12 g/dL that resolved after the dosage was decreased to 15 mg/daily.
Patients five and six, aged 82 and 72 years old respectively, were started on 15 mg/day and reached a complete clearing at week 16 which was maintained through follow-ups respectively at weeks 32 and 104. Patient five contracted a symptomatic COVID-19 infection and upadacitinib was discontinued for two weeks without loss of efficacy.
The last patient, a 75-year-old male, had a previous hepatitis-B infection. Additionally, he had stable leukopenia with lymphopenia for five years, with leukocyte and absolute lymphocyte counts of 3000 cells/uL and 510 cells/uL, respectively. Given his unsuccessful treatment with dupilumab and his stable hematologic condition without clinical symptoms, he was started on upadacitinib 30 mg following the approval of his hematologist. The dose was halved after 16 weeks due to disease stabilization. After 32 weeks, he discontinued the treatment due to complete remission of atopic dermatitis. No significant reduction in leukocytes or lymphocytes was observed during the follow-up period.
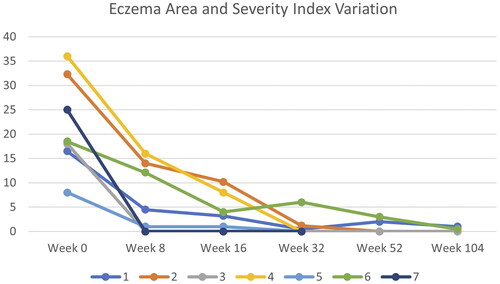
illustrates the changes in EASI scores during the follow-up period. The scores of all patients significantly improved over time. We observed minor alterations in cholesterol, triglycerides, transaminases, and creatinine phosphokinase in four patients and mild infections in two patients. There were no severe adverse events that lead to the discontinuation of the drug.
Figure 1. Eczema Area and Severity Index (EASI) Variation during follow-up observation. Seven patients reached week 32, four patients reached week 52 and three patients reached week 104.
Despite the small sample size, our findings suggest that upadacitinib can be a safe alternative for treating moderate-to-severe AD in older adults. Moreover, our findings are in line with recent molecular findings about adult-onset AD that suggest the use of a drug with a broader molecular target beyond Th2-cytokines. Further, longitudinal studies are needed to confirm its safety and effectiveness in the elderly population.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All patients received Upadacitinib as in good clinical practice, in accordance with European guidelines. All included patients had provided written consent for a retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Acknowledgements
None.
Disclosure statement
L. Gargiulo has received research grants from Almirall. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly and Boehringer Ingelhei. P. Facheris has been a consultant for Eli Lilly. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi‐Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to disclose.
Data availability statement
Data is available on request from the authors.
Additional information
Funding
References
- Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol. 2018;36(5):1–3. doi: 10.1016/j.clindermatol.2018.05.007.
- Facheris P, Da Rosa JC, Pagan AD, et al. Age of onset defines two distinct profiles of atopic dermatitis in adults. Allergy. 2023;78(8):2202–2214. doi: 10.1111/all.15741.
- Gargiulo L, Piscazzi F, Ibba L, et al. Dupilumab for the treatment of atopic dermatitis of the elderly: a real-life 52-week experience. J Dermatolog Treat. 2023;34(1):2192840. doi: 10.1080/09546634.2023.2192840.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi: 10.1111/jdv.18345.
- Gargiulo L, Ibba L, Cortese A, et al. Real-life effectiveness and safety of upadacitinib in adults and adolescents with moderate-to-severe atopic dermatitis: a single-center 16-week study. Dermatol Ther. 2023;13(2):651–660. doi: 10.1007/s13555-022-00882-z.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4.Erratum in: lancet. 2021 Jun 19;397(10292):2336. Erratum in: lancet. 2021 Aug 28;398(10302):746.
- Rinvoq_EPAR - Product Information as approved by the CHMP on 23. January 2023. Available from https://www.ema.europa.eu/en/documents/referral/rinvoq-epar-product-information-approved-chmp-23-january-2023-pending-endorsement-european_en.pdf.

