Abstract
Background: Dupilumab has been shown to be a safe and effective drug for the treatment of atopic dermatitis (AD) in children from 6 months to 11 years in randomized clinical trials. Aim: The aim of this real-life study was to determine the effectiveness in disease control and safety of dupilumab at W52 in moderate-to-severe AD children aged 6-11 years.
Methods: All data were collected from 36 Italian dermatological or paediatric referral centres. Dupilumab was administered at label dosage with an induction dose of 300 mg on day 1 (D1), followed by 300 mg on D15 and 300 mg every 4 weeks (Q4W). Treatment effect was determined as overall disease severity, using EASI, P-NRS, S-NRS and c-DLQI at baseline, W16, W24, and W52. Ninety-six AD children diagnosed with moderate-to-severe AD and treated with dupilumab were enrolled.
Results: Ninety-one (94.8%) patients completed the 52-week treatment period and were included in the study. A significant improvement in EASI score, P-NRS, S-NRS and c-DLQI was observed from baseline to weeks 16, 24 and 52.
Conclusions: Our real-life data seem to confirm dupilumab effectiveness and safety in paediatric patients. Moreover, our experience highlighted that patients achieving clinical improvement at W16 preserved this condition over time.
Dupilumab has been shown to be a safe and effective drug for the treatment of atopic dermatitis (AD) in children from 6 months to 11 years in randomized clinical trials (Citation1–4). However, there are only a few real-world studies limited to up to week (W) 24 of treatment (Citation5–9). Herein, we report a retrospective, observational, real-life experience aimed to determine the effectiveness in disease control and safety of dupilumab at W52 in moderate-to-severe AD children aged 6–11 years. The patients had been treated through a Managed Access Programme (MAP) run by Sanofi-Regeneron® from May to December 2022. MAP, also known as drug compassionate use, is a program under which investigational medicines, or medicines with ongoing marketing authorization may be used to treat patients who cannot be enrolled in an ongoing clinical trial.
A retrospective chart review was conducted through electronic medical records of each patient from 36 Italian dermatological or pediatric referral centers. Inclusion criteria were: AD patients aged from 6 to 11 years; disease inadequately controlled with topical conventional approved therapies and defined according to the following scores: (i) Eczema Area and Severity Index (EASI, a tool combining the assessment of the severity of lesions and the area affected into a single total score in the range 0 (no disease) to 72 (maximal disease) ≥24 or <24 with at least one of the following clinical characteristics: (i) involvement of sensitive areas (face, neck, hands, genitals); (ii) Pruritus Numerical Rating Scale (P-NRS, an 11-point scale used by subjects to rate their worst itch severity over the past 7 days with 0 indicating ‘no itch’ and 10 indicating ‘worst itch imaginable’.) ≥7; (iii) Children’s Dermatology Life Quality Index (c-DLQI, a 10-item validated questionnaire used to assess the impact of AD disease symptoms and treatment on quality of life) score ≥10. The outcomes of the study were the evaluation of efficacy and safety profile of dupilumab in a 52-week real-life experience in children aged 6–11 years.
Dupilumab was administered at label dosage with an induction dose of 300 mg on day 1 (D1), followed by 300 mg on D15 and 300 mg every 4 weeks (Q4W). A dose increase to 200 mg Q2W was possible at W16 in patients with a partial or unsatisfactory clinical response at W16 (failure to achieve at least EASI50). Patients were allowed to use topical treatments [moisturizers, topical corticosteroids (TCs) and/or calcineurin inhibitors (TCIs)] during the observation period.
Baseline data included: sex, age, body mass index, mean age at AD onset, clinical phenotype of AD, prior therapies, family history regarding AD, comorbidities, and concomitant medications. Treatment effect was determined as overall disease severity, using EASI, P-NRS, sleep NRS (S-NRS), and c-DLQI at baseline, W16, W24, and W52. The effectiveness of dupilumab was evaluated at each time point by assessing the patients’ percentages achieving 50, 75, and 90% improvement in EASI (EASI 50, EASI 75, and EASI 90) from baseline and by assessing the mean percentage reduction of EASI, P-NRS, S-NRS, and c-DLQI, from baseline to W16, W24, and W52 (). Adverse events (AEs) and concomitant medications were routinely recorded at each visit. Quantitative variables were expressed as the mean and standard deviation (SD), while qualitative variables as frequencies and percentages. Graph Pad Prom software (v 8.0; Graph Pad software Inc. La Jolla, CA, USA) was used for all statistical analyses. The statistical significance of changes before and after treatment was analyzed using the Fisher’s exact test or Mann–Whitney test. A p value of <0.05 was considered statistically significant. We used the Scheffe post-hoc test to make all possible contrasts between group means. The analysis was conducted by using multiple imputation for missing data, with an analysis of covariance (ANCOVA) model with treatment as a fixed effect and baseline SDI value and relevant baseline value as continuous covariates. Data of all the patients were examined and analyzed by the same dermatologists (C.P. and M. N).
Table 1. Outcome of therapy with dupilumab at week 16, week 24 and week 52.
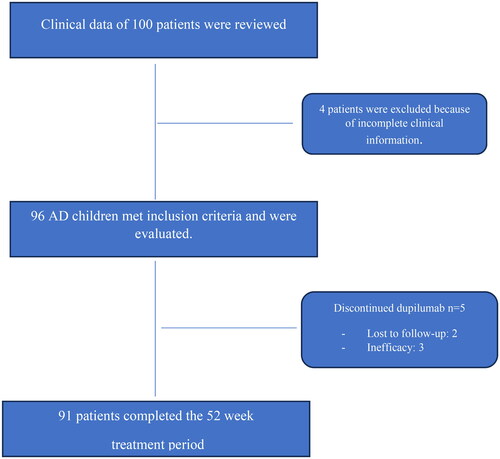
The clinical data of 100 patients were reviewed, of which 4 patients were excluded because of incomplete clinical information. Ninety-six AD children [males: 50 (52.1%)] diagnosed with moderate-to-severe AD and treated with dupilumab during the reference period were evaluated. The mean age was 8.22 ± 3.96 years and the average disease duration was 7.15 ± 5.08 years. AD started within the first year of age in 63/96 (65.6%), between 2 and 6 years in 27/96 (28.1%), and after 6 years in 6/96 (6.25%) patients, respectively. At baseline, 58/96 (60.4%) patients had a family history of AD; 50/96 (52%) reported at least 1 concurrent atopic comorbidity, with allergic rhinitis accounting for 45.8% (44/96), asthma for 33.3% (32/96), conjunctivitis for 12.5% (12/96), and food allergy for 6.25% (6/96) of cases. Other comorbidities included attention deficit hyperactivity disorder (7/96; 7.3%), celiac disease (5/96; 5.2%), chronic urticaria (2/96; 2.1%), von Willebrand disease (1/96; 1.04%), thalassemia major (1/96; 1.04%), Marfan syndrome (1/96; 1.04%), and primary immunodeficiency associated with Hartnup disease (1/96; 1.04%). The baseline demographic and clinical characteristics of patients are reported in .
Table 2. Demographic and clinical characteristics of the patient population (n =96).
Ninety-one (94.8%) patients completed the 52-week treatment period and were included in the effectiveness analysis (). Five out of 96 (5.2%) patients discontinued dupilumab: 2 lost to follow-up after 24 weeks, and 3 for drug ineffectiveness after 16 weeks (mean EASI: 29.21 + 2.76) (). The overall drug survival rate at W52 was 94.8%. No patient required dupilumab dose adjustment.
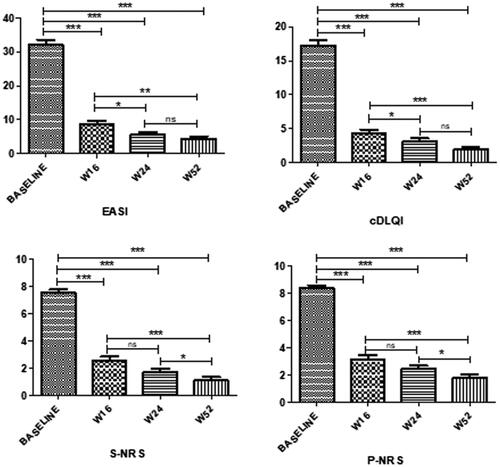
Dupilumab treatment led to a significant reduction of mean EASI score from baseline to weeks 16, 24, and 52 (p < 0.0001 for all the assessments), and from W16 to W24 (p < 0.01) and 52 (p < 0.001); no statistically significant difference was instead observed from W24 to W52 (). Compared to baseline, the mean EASI percentage reduction was 75.5% at W16, 82.2% at W24, and 88.8% at W52, respectively. C-DLQI score demonstrated a significant (p < 0.0001) decrease from baseline at all timepoints (mean percentage reduction of 74.9% at W16, 77.3% at W24, and 88.8% at W52), from W16 to W24 (p < 0.01), and W52 (p < 0.0001). There were no differences in c-DLQI between W24 and W52. Likewise, a significant improvement was observed in both mean P-NRS and S-NRS from baseline at all timepoints (p < 0.0001). The mean percentage reduction from baseline to W16, W32, and W52 was 62.4%, 70.7%, and 78.9% for P-NRS, and of 66%, 77.2%, and 85.7% for S-NRS.
After 52 weeks of treatment, the proportion of patients achieving EASI50, EASI75, and EASI90 was 95.6% (n = 87/91), 86.8% (n = 79/91), and 42.9% (n = 39/91), respectively. During the follow-up period a reduction of TCs use was observed in our patients, to control AD clinical flares. No systemic medications were allowed during the observation period in addition to dupilumab therapy. AEs were reported by 26/96 (27.08%) children, but only 14 (14.58%) of these experienced drug related AEs. No serious AEs were recorded and none of the children discontinued dupilumab. In particular, conjunctivitis was diagnosed in 8/96 patients (8.3%); the first episode occurred on average after 10.5 ± 7.71 weeks and healed within W32 of dupilumab treatment, after corticosteroid or tacrolimus eye drops treatment. Injection site reactions were reported by 6 children (6/96; 6.25%) and in all cases these appeared within the first week after injection, with spontaneous resolution in a few days without any treatment. Ten (10.4%) patients reported asymptomatic SARS-COV-2 infection (diagnosed by molecular-PCR swab), but continued dupilumab with a regular course of the infection. None of the other common AEs reported in adults (such as facial redness or psoriatic eruption) was observed in our patients.
This study seems to suggest that dupilumab is efficacious in term of EASI-50, EASI-75, and EASI-90 at 52 weeks in AD children aged from 6 to 11 years. A double-blind, 16-week, phase III trial (LIBERTY AD PEDS; ClinicalTrials.gov identifier: NCT03345914) showed dupilumab efficacy and safety in patients from 6 to 11 years with a percentage of 69.7, 67.2, and 26.8% of patients receiving dupilumab Q4W, dupilumab Q2W or placebo reaching EASI-75 at W16, respectively (Citation1). Of note, phase III open-label extension data at 52 weeks are not available for children. In our cohort, dupilumab rapidly improved AD symptom severity with a statistically significant difference in EASI as early as baseline to W16, and then to W24 and W52 (). Interestingly, a continuous and significant improvement of EASI has also been observed from W16 to W52 (). Moreover, our experience highlighted that patients achieving clinical improvement at W16 maintained this condition over time. In particular, the mean values achieved were substantially lower than those required to access dupilumab therapy and found at baseline. Similarly, improvements in all areas of symptoms, including pruritus and sleep disorders, were observed from baseline to all timepoints, and from W16 to W52. Regarding AEs, the rate of conjunctivitis and injection site reaction found in our study (8.3 and 6.25%, respectively) is in line with what reported from clinical trials (Citation10). This data confirms the good safety of dupilumab also in long-term treatment of children AD, as previously reported in similar adults and adolescents studies (Citation11–13). The efficacy and safety of the drug also account for the high survival of dupilumab treatment.
Figure 2. Outcome at week 16, week 24, and week 52 of therapy with dupilumab in 91 Childrem 6-11 years of age with moderate-to-severe atopic dermatitis.
EASI: Eczema Area and Severity Index; S-NRS: Sleep Numerical Rating Scale; P-NRS: Pruritus Numerical Rating Scale; c-DLQI: Children’s Dermatology Life Quality Index; W: week of dupilumab treatment; ***p < 0.0001; **p < 0.001; *p < 0.005; ns: not significant.
Our study has several limitations. First, it was a retrospective cohort study and, although the cohort was specially selected to include only patients with AD and the use of dupilumab, selection bias cannot be ruled out. Some patients were lost to follow-up. Due to the limited sample size, it was challenging to analyze potential factors affecting the therapeutic effect of dupilumab in children. Finally, monitoring bias is a concern as investigators may have seen patients more frequently for the according to the MAP program, which may improve patient adherence.
Ethical approval
The patients in this manuscript have given written informed consent to publication of their case details.
Author contributions
Conceptualization: Cataldo Patruno, Gabriella Fabbrocini, Valeria Boccaletti, Cristiana Colonna, Riccardo Cavalli, Iria Neri, Michela Ortoncelli, Donatella Schena, Luca Stingeni, Katharina Hansel, Vincenzo Piccolo, Veronica Di Brizzi, Concetta Potenza, Ersilia Tolino, Luca Bianchi, Sara Manti, Rocco De Pasquale, Vito Di Lernia, Lucia Caminiti, Elena Galli, Paola Coppo, Andrea Chiricozzi, Clara De Simone, Cristina Guerriero, Fabrizio Giuseppe Amoruso, Eugenio Provenzano, Salvatore Leonardi, Amelia Licari, Gian Luigi Marseglia, Antonino Palermo, Sabrina Di Pillo, Daniele Russo, Viviana Moschese, Vincenzo Patella, Tiziana Peduto, Caterina Ferreli, Paola Zangari; Federica Veronese, Samantha Federica Berti, Michaela Gruber, Elena Pezzolo, Stefania Termine, Rosanna Satta, Federica Dragoni, Maria Esposito, Maria Concetta Fargnoli, Ylenia Vallone, Giuseppe Lauletta, Vincenzo Picone, Maddalena Napolitano. Methodology: Cataldo Patruno, Maddalena Napolitano. Formal analysis and investigation: Cataldo Patruno, Giuseppe Lauletta, Vincenzo Picone, Maddalena Napolitano. Writing-review and editing: Cataldo Patruno, Gabriella Fabbrocini, Valeria Boccaletti, Cristiana Colonna, Riccardo Cavalli, Iria Neri, Michela Ortoncelli, Donatella Schena, Luca Stingeni, Katharina Hansel, Vincenzo Piccolo, Veronica Di Brizzi, Concetta Potenza, Ersilia Tolino, Luca Bianchi, Sara Manti, Rocco De Pasquale, Vito Di Lernia, Lucia Caminiti, Elena Galli, Paola Coppo, Andrea Chiricozzi, Clara De Simone, Cristina Guerriero, Fabrizio Giuseppe Amoruso, Eugenio Provenzano, Salvatore Leonardi, Amelia Licari, Gian Luigi Marseglia, Antonino Palermo, Sabrina Di Pillo, Daniele Russo, Viviana Moschese, Vincenzo Patella, Tiziana Peduto, Caterina Ferreli, Paola Zangari; Federica Veronese, Samantha Federica Berti, Michaela Gruber, Elena Pezzolo, Stefania Termine, Rosanna Satta, Federica Dragoni, Maria Esposito, Maria Concetta Fargnoli, Ylenia Vallone, Giuseppe Lauletta, Vincenzo Picone, Maddalena Napolitano. Supervision: Cataldo Patruno. All authors read and approved the final version of the manuscript.
Disclosure statement
Cataldo Patruno acted as investigator, speaker, consultant and advisory board member for AbbVie, Amgen, Eli Lilly, LEO Pharma, Novartis, Pfizer, Pierre Fabre and Sanofi; Gabriella Fabbrocini has been principal investigator in clinical trials sponsored by and/or has received personal fees from AbbVie, Abiogen, Almirall, Celgene, Eli Lilly, LEO Pharma, Novartis, Sanofi and UCB; Luca Stingeni has been principal investigator in clinical trials sponsored by and/or and has received personal fees for participation in advisory boards from AbbVie, LEO Pharma, Novartis and Sanofi, outside the submitted work; Katharina Hansel reports personal fees from AbbVie and Novartis, outside the submitted work; Paola Coppo received support for attending meetings and/or travel from Pierre Fabre and Sanofi; Andrea Chiricozzi has served as advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Bristol Myers Squibb, Leo Pharma, Lilly, Janssen, Novartis, Pfizer and Sanofi Genzyme; Clara De Simone has served as advisory board member and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Abbvie, Almirall,Eli Lilly, LeoPharma, Novartis, Janssen, Sanofi, UCB Pharma, Amgen, Boehringer Ingelheim; Viviana Moschese has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi-Genzyme; Caterina Ferreli has received support for meetings and/or travel from Novartis, Lilly, Abbvie, Almirall, Galderma, Leo Pharma, Pierre Fabre, Sanofi, Sun Pharma, Janssen; Maria Esposito acted as speaker, consultant and advisory board member for Sanofi, AbbVie, LEO Pharma; Maria Concetta Fargnoli has been principal investigator in clinical trials sponsored by and/or has received personal fees from AbbVie, Sanofi, Galderma, Pfizer; Maddalena Napolitano acted as speaker, consultant and advisory board member for Sanofi, AbbVie, LEO Pharma, Amgen, Pierre Fabre, and Eli Lilly. No potential conflict of interest was reported by the author(s).
Data availability statement
Data are reported in the current study.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1–6. doi: 10.1016/j.jaad.2020.06.054.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184(5):857–870. PMCID: PMC8247037. doi: 10.1111/bjd.19460.
- Paller AS, Siegfried EC, Cork MJ, et al. Laboratory safety from a randomized 16-week phase III study of dupilumab in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. Paediatr Drugs. 2023;25(1):67–77. doi: 10.1007/s40272-022-00553-8.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10356):908–919. doi: 10.1016/S0140-6736(22)01539-2.
- Yang N, Ye Y, Shao J, et al. Efficacy of dupilumab in children 6 months to 11 years old with atopic dermatitis: a retrospective real-world study in China. Dermatitis. 2023. doi: 10.1089/derm.2022.0069
- Pagan AD, David E, Ungar B, et al. Dupilumab improves clinical scores in children and adolescents with moderate to severe atopic dermatitis: a real-world, single-center study. J Allergy Clin Immunol Pract. 2022;10(9):2378–2385. doi: 10.1016/j.jaip.2022.06.014.
- Lasek A, Bellon N, Mallet S, et al. Effectiveness and safety of dupilumab in the treatment of atopic dermatitis in children (6-11 years): data from a french multicentre retrospective cohort in daily practice. J Eur Acad Dermatol Venereol. 2022;36(12):2423–2429. doi: 10.1111/jdv.18450.
- Colonna C, Zussino M, Monzani NA, et al. Treatment of children from 6 to 11 years old affected by moderate-severe atopic dermatitis with dupilumab: a first perspective from a single-center real-life experience in Italy. J Eur Acad Dermatol Venereol. 2022;36(6):e478–e480. doi: 10.1111/jdv.17963.
- Napolitano M, Fabbrocini G, Neri I, et al. Dupilumab treatment in children aged 6–11 years with atopic dermatitis: a multicentre, real-life study. Paediatr Drugs. 2022;24(6):671–678. doi: 10.1007/s40272-022-00531-0.
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis: results through week 52 from a phase III Open-Label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23(3):365–383. doi: 10.1007/s40257-022-00683-2.
- Stingeni L, Bianchi L, Antonelli E, et al. A 52-week update of a multicentre italian real-world experience on effectiveness and safety of dupilumab in adolescents with moderate-to-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37(3):e384–e388. doi: 10.1111/jdv.18648.
- Silverberg JI, Lynde CW, Abuabara K, et al. Efficacy and safety of dupilumab maintained in adults ≥60 years of age with moderate-to-Severe atopic dermatitis: analysis of pooled data from four randomized clinical trials. Am J Clin Dermatol. 2023;24(4):673. doi: 10.1007/s40257-022-00754-4.
- Patruno C, Fabbrocini G, Longo G, et al. Effectiveness and safety of long-term dupilumab treatment in elderly patients with atopic dermatitis: a multicenter real-life observational study. Am J Clin Dermatol. 2021;22(4):581–586. doi: 10.1007/s40257-021-00597-5.