Abstract
The purpose of the article
Nail psoriasis is a refractory disease that affects 50–79% skin psoriasis patients and up to 80% of patients with psoriatic arthritis (PsA). The pathogenesis of nail psoriasis is still not fully illuminated, although some peculiar inflammatory cytokines and chemokines seems to be the same as described in psoriatic skin lesions. Treatment of nail psoriasis still with challenge and should be individualized. Upadacitinib, an oral highly selective JAK1 inhibitor, has been approved for PsA treatment. Whether it has the therapeutic advantages for nail psoriasis.
Results
We report a case of a patient with nail psoriasis who responded well to upadacitinib therapy at a dose of 15mg once daily for 5 months. In addition, we reviewed the literature and compared the current treatment efficiency in the treatment of nail psoriasis. The therapeutic effects of JAK inhibitors for nail psoriasis may involve downstream cytokines, such as I IL-6, IL-10, and IL-23.
Conclusion
Upadacitinib may be a promising therapeutic option for patients with severe nail psoriasis.
Nail involvement is observed in up to 80% of individuals affected by psoriasis and is the only manifestation in 6% of patients with psoriasis (Citation1). Nail psoriasis may be a risk factor for the development of psoriatic arthritis (PsA) and can impair function and reduce quality of life. Nail psoriasis also presents a therapeutic challenge; 11–13% of patients have recalcitrant psoriasis (Citation2). Biologics are a promising option for nail psoriasis; 50–70% patients improve (Citation3). Herein we report a patient with a Nail Psoriasis Severity Index (NAPSI) of 80. The nail psoriasis caused severe discomfort and impaired her ability to study and work. We treated the nail psoriasis with upadacitinib, a Janus-activated kinase (JAK) inhibitor and achieved a good response within 4 months.
Case report
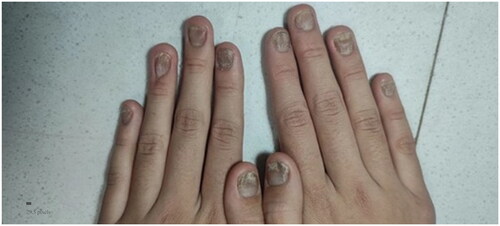
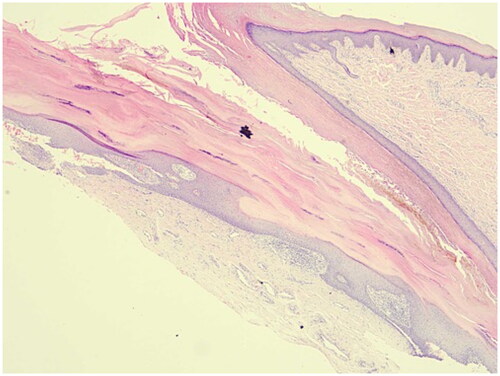
A 13-year-old female had an 8-year history of progressive nail distortion without a history of trauma, skin lesions, or joint pain. Previous therapies included oral Chinese traditional medicine and topical glucocorticoid gels, both of which were ineffective. The physical examination did not reveal erythematous and scaly plaques on the body. All of the fingernails on both hands were dystrophic, with different degrees of trachyonychia, longitudinal striations, and discoloration (). The toenails were also affected. The clinical features were suggestive of nail psoriasis or nail dystrophy. A longitudinal nail biopsy was obtained, which showed hyperkeratosis, parakeratosis, and neutrophils in the nail plate, suggestive of nail psoriasis (). Infection was excluded by three fungal and bacterial cultures. NAPSI was 80. The patient had severe discomfort which impaired her ability to study and work. The Dermatology Life Quality Index was 20.
Figure 1. Nails psoriasis at both hands. All of the fingernails on both hands were dystrophic, with different degrees of trachyonychia, longitudinal striations, and discoloration.
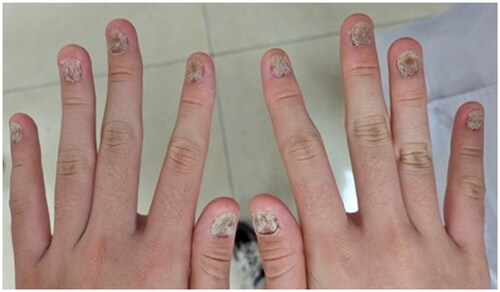
Figure 2. Nail biopsy. A nail biopsy showed hyperkeratosis, parakeratosis, and neutrophils in the nail plate, suggestive of nail psoriasis (HE staining: ×40).
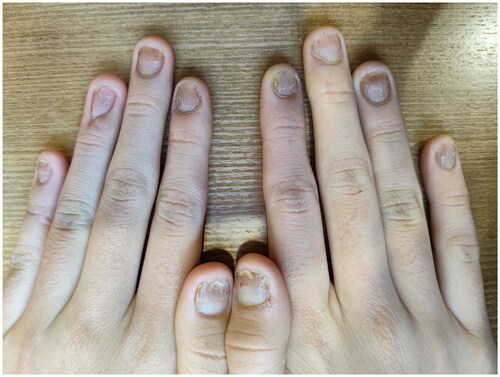
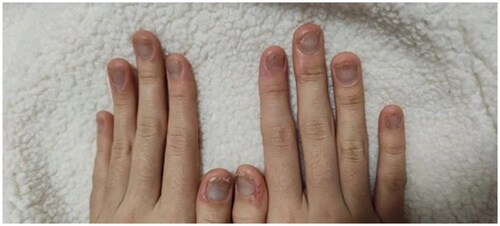
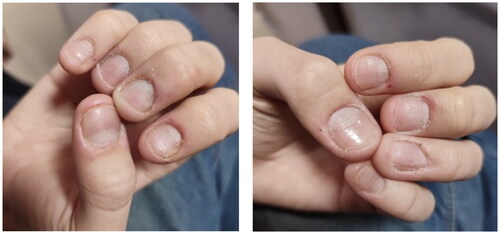
After screening for small molecule inhibitor treatment, the patient was treated with upadacitinib (15 mg once daily for 5 months). Improvement was noted at 12 weeks of upadacitinib therapy (NAPSI = 30; DLQI = 15; ). The patient reported 40% subjective improvement of the affected fingernails. By week 16, the patient reported 60% subjective improvement of the affected fingernails (NAPSI = 15; DLQI = 10, ). By week 20 of treatment, all nails showed near-complete remission of the psoriatic lesions on both hands, with pitting and discoloration of one finger only (NAPSI = 6; ). These results were maintained over time at the 20- and 24-week follow-up evaluations (). The toenail lesions also resolved. In addition, we did not note any adverse events throughout the entire treatment.
Discussion
Nail involvement is estimated to affect 80–90% of patients with psoriasis at some point in their lives, even though nail involvement can represent the only manifestation of the disease (Citation3), as in our patient. Patients with nail involvement experience pain, functional impairment, and social stigma, along with a significant restriction of daily activities and quality of life. Female patients with nail psoriasis are affected more severely than male patients. Nail psoriasis is also considered a risk factor for the development of PsA (Citation3).
Management of nail psoriasis is challenging. Generally, topical therapy is difficult due to nail anatomy, the extent of treatment, and poor adherence that often results in unsatisfactory efficacy (Citation4). While biologic agents are an option, the improvement varies. When the reduction of NAPSI at week 24 is regarded as the endpoint of biologic therapy, the nail improvement varies from 55.0 to 75.7%, they rank as follows: infliximab, ixekizumab, etanercept, ustekinumab, adalimumab, and secukinumab (Citation4,Citation5).
Janus kinase (JAK) inhibitors block the intracellular signal pathway mediated by JAK and signal transducer and activator of transcription (STAT) proteins, thereby inhibiting gene transcription of proinflammatory cytokines (Citation6), such as interferon (IFN) and IL-6, IL-10, and IL-23. The IL-23/Th17 axis is a key part of the pathogenesis of psoriasis. The IL-23 receptor relies on a heterodimer of JAK2 and TYK2 for signal transduction, thus highlighting the role of JAKs in the pathogenesis of psoriasis and the therapeutic potential of JAK inhibitors (Citation7). JAK inhibitors are currently being tested as potential treatments for psoriasis. JAK inhibitors are effective, as measured by 75% improvement in Psoriasis Area and Severity Index in both phase 2 and 3 trials, and appear to be well-tolerated overall. JAK inhibitors block the signaling of key cytokines implicated in the immune response and inflammatory pathways of psoriasis. In Japanese patients, both tofacitinib 5 mg and tofacitinib 10 mg greatly improved NAPSI (Citation8). Several studies have reported administering tofacitinib, a JAK 1/3 inhibitor, to improve PsA and nail psoriasis (Citation8,Citation9), therefore, we administered upadacitinib, an oral highly selective JAK1 inhibitor, approved for PsA. In addition, upadacitinib is widely used in the treatment of dermatologic diseases and has a good safety profile, as demonstrated in phase 2 and 3 clinical trials of PsA and atopic dermatitis, respectively (Citation10,Citation11).
The differential diagnosis of nail psoriasis is nail lichen planus (NLP). Clinical criteria for diagnosis of NLP were nail plate thinning with longitudinal ridging and fissuring, with or without pterygium. Biopsy of NLP showing a bandlike lymphocytic infiltrate of the nail matrix and/or nail bed dermis together with hyperkeratosis, hypergranulosis and acanthosis of the nail matrix epithelium. What is more, NLP is potentially scarring, and the changes may be irreversible (Citation12). The JAK2 is considered as the cornerstone of the pathogenesis of NLP, which was rapidly and sustainably controlled following treatment with baricitinib in one case report (Citation13).
Notwithstanding the crucial role of JAK1 in the pathogenesis of nail psoriasis, there are no data pertaining to the use of upadacitinib in patients with nail psoriasis. Nail psoriasis was rapidly and sustainably controlled following treatment with upadacitinib in the patient reported herein, without any adverse effects. Indeed, upadacitinib may be a promising therapy for patients with severe nail psoriasis, but further clinical studies are needed to support the findings in this case.
Ethical approval
Informed written patient consent was taken from the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article.
Additional information
Funding
References
- Canal-García E, Bosch-Amate X, Belinchón I, et al. Nail psoriasis. Actas Dermosifiliogr. 2022;113(5):1–3. doi: 10.1016/j.ad.2022.01.006.
- Hjuler KF, Iversen L, Rasmussen MK, et al. Localization of treatment-resistant areas in patients with psoriasis on biologics. Br J Dermatol. 2019;181(2):332–337. doi: 10.1111/bjd.17689.
- Thomas L, Azad J, Takwale A. Management of nail psoriasis. Clin Exp Dermatol. 2021;46(1):3–8. doi: 10.1111/ced.14314.
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61(3):377–402. doi: 10.1007/s12016-021-08896-9.
- Szebényi J, Gede N, Hegyi P, et al. Efficacy of biologics targeting tumour necrosis factor-alpha, interleukin-17 -12/23, -23 and small molecules targeting JAK and PDE4 in the treatment of nail psoriasis: a network meta-analysis. Acta Derm Venereol. 2020;100(18):adv00318. doi: 10.2340/00015555-3640.
- Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther. 2020;10(1):29–42. doi: 10.1007/s13555-019-00347-w.
- Singh S, Pradhan D, Puri P, et al. Genomic alterations driving psoriasis pathogenesis. Gene. 2019;683:61–71.
- Abe M, Nishigori C, Torii H, et al. Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: subgroup analyses from a randomized, placebo-controlled phase 3 trial. J Dermatol. 2017;44(11):1228–1237. doi: 10.1111/1346-8138.13956.
- Papp KA, Menter MA, Abe M, et al. Tofacitinib, an oral janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173(4):949–961. doi: 10.1111/bjd.14018.
- Mease PJ, Lertratanakul A, Papp KA, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-Week data from the randomized controlled phase 3 SELECT-PsA 2 study. Rheumatol Ther. 2021;8(2):903–919. doi: 10.1007/s40744-021-00305-z.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4.
- Grover C, Chaturvedi UK, Reddy BN. Role of nail biopsy as a diagnostic tool. Indian J Dermatol Venereol Leprol. 2012;78(3):290–298. doi: 10.4103/0378-6323.95443.
- Pünchera J, Laffitte E. Treatment of severe nail lichen planus with baricitinib. JAMA Dermatol. 2022;158(1):107–108. doi: 10.1001/jamadermatol.2021.5082.