Abstract
Purpose
Ixekizumab is a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A approved for the treatment of moderate-to-severe plaque psoriasis. The objective of this study was to describe the real-world long-term effectiveness of ixekizumab in patients with plaque psoriasis in Italy.
Materials and Methods
A retrospective study was conducted in patients affected by moderate-to-severe plaque psoriasis who were continuously treated with ixekizumab for at least 12 months. Patient data was obtained at 4-weeks, 12-weeks and 6-, 12-, 18- and 24-months after baseline (June 2017 and September 2019) from 10 sites. Results were analyzed by complete case approach, with sensitivity analysis performed to evaluate the impact of missing data.
Results
A total of 198 patients were enrolled in the study. At Month 24, 94.3% of patients achieved PASI75 response, while 85.1 and 71.8% achieved PASI90 and PASI100, respectively; and 91.1% of the patients achieved absolute PASI score ≤2. Patients experienced psoriasis improvement at 4 weeks after starting treatment, and improvement was maintained with continued ixekizumab use. The quality of life of patients also improved significantly starting at Week 12, with sustained effect in the long term.
Conclusion
This 24-month observational cohort study confirmed that ixekizumab is effective in the long-term management of patients with moderate-to-severe plaque psoriasis.
Introduction
Psoriasis is a common, chronic, inflammatory skin disorder that affects approximately 125 million people worldwide, with a prevalence highly variable across countries, ranging from 0.9 to 8.5% (Citation1,Citation2). Psoriasis prevalence among the adult Italian population is estimated to be 2.7% (Citation3). The most common form is plaque psoriasis, which occurs in 85–90% of patients (Citation4). Plaque psoriasis may be associated with a heavy psychosocial burden which can significantly impact on the quality of life of patients, and is often accompanied by several comorbidities, such as psoriatic arthritis, metabolic syndrome, cardiovascular diseases, and nonalcoholic steatohepatitis (Citation5,Citation6).
Common first-line treatments for moderate-to-severe plaque psoriasis are phototherapy and conventional systemic therapies. However, the advent of biologics has changed the paradigm of treatment in recent years, as they usually attain higher efficacy compared with conventional systemic agents, along with greater patient satisfaction and compliance (Citation7–10). Ixekizumab is a high-affinity monoclonal antibody that selectively targets interleukin (IL)-17A, thus inhibiting the master cytokine mediating the key inflammatory loops driving the development of psoriasis phenotype (Citation11). The efficacy and safety of ixekizumab were assessed and established in three randomized, double-blind, placebo-controlled phase III studies in adult patients with moderate-to-severe plaque psoriasis who were candidates for phototherapy or systemic therapy (UNCOVER-1, UNCOVER-2, and UNCOVER-3) (Citation12–15). Long-term extension of these trials also demonstrated efficacy and safety of ixekizumab up to 5 years in psoriasis patients (Citation16,Citation17).
Nonetheless, there are only few studies assessing the long-term effectiveness of ixekizumab in a real-life setting. LOTIXE is an Italian observational study aimed at obtaining real-world data on patient profiles, treatment patterns and long-term effectiveness of ixekizumab in a cohort of adult patients with moderate-to severe plaque psoriasis who received ixekizumab for at least 12 months.
Materials and methods
This is a retrospective, multicenter, Italian, observational cohort study based on medical review of patient charts. The study included patients who were at least 18 years of age with a diagnosis of moderate-to-severe plaque psoriasis who were continuously being treated with ixekizumab for at least 12 months, as per approved label in real clinical practice. Patients who received systemic treatments in combination with ixekizumab, and patients who received treatment only for psoriatic arthritis were excluded from this study. The study was conducted with medical chart review method in 10 Italian centers, and the data was obtained by trained investigators and filed in a centralized database for analysis, through a period from January 2022 to May 2022. The time points of interest were 4-weeks, 12-weeks and 6-, 12-, 18- and 24-months after the start of treatment (baseline), which occurred between June 2017 and September 2019.
The primary objective of the study was to report and evaluate a 75% or greater reduction in Psoriasis Area Severity Index (PASI 75) response rates at 24 months. Secondary objectives were to report and assess PASI 75, PASI 90, and PASI 100 response rates at each time point of interest, the absolute PASI scores and changes from baseline at each time point of interest, the proportion of patients who achieved absolute low PASI scores at each time point of interest, and the Dermatology Life Quality Index (DLQI) scores at each time point of interest. In addition, ixekizumab utilization patterns and patient demographic and characteristics were also retrieved. Continuous data are summarized by mean and standard deviation (SD). Categorical data are presented by absolute and relative frequencies (n and %) or contingency tables. 95% Confidence Intervals (CIs) are provided when applicable. Results are presented as complete case analysis, and additional sensitivity analysis has been performed applying multiple imputation and last observation carried forward (LOCF) methods to evaluate the impact of incompleteness of the data for the primary endpoint. All statistical analyses were performed by means of SAS® release 9.4 or later (SAS Institute, Inc., Cary, NC, USA).
The study was conducted in accordance with the Declaration of Helsinki, Good Pharmacoepidemiology Practices (GPPs), and the regulations of the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA). The study was approved by the Ethical Review Board of the local committee of all participating sites, and informed consent was obtained from each participant before inclusion in the study.
Results
A total of 201 patients with moderate-to-severe plaque psoriasis were screened, and 198 patients were enrolled in this retrospective observational study. The reasons for screen failures included receipt of systemic treatment in combination with ixekizumab (1 patient), and no available source documents or incomplete source documents necessary for primary endpoint evaluation (2 patients). Among the enrolled patients, 70.2% (139 patients) were male, and the mean age was 50.0 years at baseline. The average weight, height and body mass index at baseline were 79.9 kg, 171.2 cm, and 27.3 kg/m2, respectively. Patient characteristics at baseline are summarized in . Less than half of the patients (86 patients, 43.4%) had at least one comorbid condition upon study entry, including hypertension, psoriatic arthritis, depression, and type 2 diabetes (Supplementary Table 1).
Table 1. Baseline patient characteristics.
Among the observed patients, 102 patients (51.5%) had a diagnosis of severe plaque psoriasis as reported by the clinicians, and the remaining 96 (48.5%) had moderate disease. Disease localization in special areas was observed in a limited proportion of patients (). The mean age at psoriasis diagnosis was 35.3 years, while the mean disease duration was 14.7 years at baseline. Most of the observed patients (179, 90.4%) had at least one prior anti-psoriatic treatment (including conventional systemic, biologics and topical agents); whereas 136 patients were naïve to biologics.
Among the 198 observed patients, 174 were evaluable for the primary endpoint according to a complete case analysis (174 patients had available PASI information at both baseline and Month 24, but the number of patients who were still on treatment at Month 24 was higher, as demonstrated by the overall ixekizumab drug retention at the end of study). Out of 174 patients, 164 patients achieved PASI 75 at Month 24, resulting in a PASI 75 response rate of 94.3% [CI: 90.8; 97.7]. After imputing missing values using the Multiple Imputation (MI) procedure, this proportion was similar; among 198 evaluable patients, the combined proportion of 20 imputed datasets was equal to 94.4% [91.0, 97.8]. Alternatively, with the Last Observation Carried Forward (LOCF) approach, 193 patients were evaluable (5 patients were excluded because PASI baseline value was not available), resulting in a proportion of patients achieving PASI75 equal to 94.3% [CI: 91.0, 97.6]. Patients were also evaluated for PASI 75, PASI 90, and PASI 100 responses at various timepoints, and as early as Week 4, 66.4 37.9, and 27.6% achieved PASI 75, PASI 90, PASI 100, respectively. At the end of the study period at Month 24, 94.3, 85.1, and 71.8% of the patients achieved PASI 75, PASI 90, and PASI 100, respectively. The proportion of patients achieving these targets clearly increased over time from Week 4 to Month 6, and then remained fairly constant from Month 6 to Month 24, as indicated in .
Table 2. Proportion of patients achieving PASI 75, PASI 90, and PASI 100.
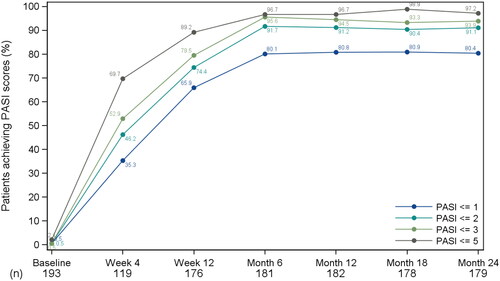
Among the observed patients, the mean PASI score at baseline was 18.6 (SD = 7.60), and these patients obtained an average of 78.7% improvement by Week 4 and 93.6% improvement by Month 24. As early as Week 4, the proportion of patients achieving absolute PASI score of ≤1, ≤2, ≤3, and ≤5 increased to 35.3, 46.2, 52.9, and 69.8%, respectively. The proportions of patients achieving these absolute PASI score targets increased over time, with about 80% of patients achieving PASI ≤1, and over 90% of patients achieving PASI ≤2 at Month 6. At Month 24, the proportion of patients achieving absolute PASI score of ≤1, ≤2, ≤3, and ≤5 was 80.5, 91.1, 93.9, and 97.2%, respectively, as shown in .
Figure 1. Line graph of proportions of patients who achieved absolute PASI scores of ≤1, ≤2, ≤3, and ≤5; observed patients. PASI: Psoriasis Area Severity Index. Proportions were computed on the enrolled population with non-missing PASI. Baseline is the baseline visit (i.e., Week 0).
Mean baseline DLQI score was 17.4 (SD = 7.28), which corresponded to a ‘very large effect on patient’s life.’ By Week 12, the mean change from baseline in DLQI was −14.6, and these patients had a mean DLQI score of 1.9, corresponding to a ‘small effect on patient’s life.’ From Week 12 to Month 24, the mean change from baseline in DLQI score ranged from −14.6 to −17.3, suggesting that patients experienced a great improvement in quality of life as early as Week 12 that was sustained in the long-term.
The overall ixekizumab drug retention was high, as at the end of study at Month 24, 190 patients (96.0%) were undergoing treatment, whereas 8 (4.0%) patients discontinued treatment due to lack or loss of effectiveness.
Subgroup analysis: biologic-naïve vs. biologic-experienced
There were 136 biologic-naïve patients (68.7%) and 62 biologic-experienced patients (31.3%) in this study, and the proportion of patients achieving PASI 75, PASI 90 and PASI 100 was similar between biologic-naïve and biologic-experienced patients at each time point. The proportion of patients who achieved PASI 75 at Month 24 was 95.0% for biologic-naïve patients and 92.7% for biologic-experienced patients. The proportion of patients with PASI 90 at Month 24 was 84.0% for biologic-naïve patients and 87.3% for biologic-experienced patients. Lastly, complete clearance of psoriasis symptoms (PASI 100) was achieved by 70.6% of biologic-naïve patients, and by 74.6% of biologic-experienced patients at Month 24. Details of the PASI 75, PASI 90, and PASI 100 responses for biologic-naïve and biologic-experienced patients may be found in .
Table 3. Proportion of patients achieving PASI 75, PASI 90, and PASI 100 at various time points; stratified by previous biologics exposure.
The average absolute PASI scores at baseline were 19.4 and 16.9 in biologic-naïve and biologic-experienced patients, respectively. At Week 4, biologic-naïve patients achieved an average of 78.2%-improvement from baseline, whereas biologic-experienced patients achieved an average of 80.1%-improvement from baseline. Biologic-naïve patients achieved a 94.2%-improvement from baseline at Month 24, and biologic-experienced patients achieved a 92.3%-improvement from baseline at Month 24. Apart from Week 4, the % of improvement in PASI score was slightly higher in biologic-naïve patients at all time points.
Discussion
This retrospective observational study confirmed that ixekizumab is effective in improving psoriasis symptoms and quality of life up to 24 months among a responder enriched population of patients with moderate-to-severe plaque psoriasis who had been treated for at least 12 months. Our study results showed that patients treated with ixekizumab achieved progressive absolute PASI reduction up to 6 months, and that the patient cohort also exhibited progressive PASI 75, PASI 90, and PASI 100 response rates up to Month 6, demonstrating that the effect of ixekizumab reached a plateau after approximately six months of treatment. It was also evident that ixekizumab has a rapid effect, as patients showed an average reduction in PASI score of 15.3 points after 4 weeks of treatment, which corresponded to a 78.7% improvement in skin clearance; and that more than half of the patients (66.4% of patients) in the responder enriched population attained PASI 75 as early as Week 4. In addition, it can be observed that ixekizumab response is maintained with continuous treatment, as in our study the PASI response rates remained relatively high from Month 6 to Month 24; and the absolute PASI scores of patients remained consistently low from Month 6 to the end of study at Month 24.
Our study also demonstrated that PASI 90 and PASI 100 responses may be achieved in a higher percentage of patients treated with ixekizumab in a real-world setting. In our study, at Week 12, 69.8% of patients achieved PASI 90 response, and 55.8% of the patients achieved PASI 100 response. This is congruent with the results published in Damiani et al. (Citation18), a prospective observational study of 47 psoriatic patients followed for 20 weeks, in which 76% of patients achieved PASI 90 and 55% of patients achieved PASI 100 after 12 weeks of ixekizumab treatment. A retrospective, observational study of patients from seven Italian centers (sample size of 201 patients) also confirmed the effectiveness of ixekizumab in treating moderate-to-severe plaque psoriasis in adults, as 90, 72, and 57% of patients achieved PASI 75, 90, and 100 responses, respectively, after 24 weeks of therapy (Citation19). A retrospective observational study of 11 Italian centers on 779 patients also showed sustained effectiveness until 4 years of observation, with a median PASI of 0.58 after 2 years of treatment and a consistent reduction of DLQI (Citation20). Similar results were observed in another real-world, retrospective study involving seven Spanish centers (sample size of 100 patients), where 82.9, 58.5, and 41.5% of patients achieved PASI 75, 90, and 100 responses, respectively, after 52 weeks of treatment; and 85% of patients maintained ixekizumab treatment at the end of the study (Citation21). In a retrospective observational study of a small cohort of Canadian patients with moderate-to-severe plaque psoriasis (sample size of 38 patients), a rapid response to treatment was also observed as early as week 4 (Citation22). In addition, Week 12 primary outcomes from an international observational psoriasis study (Psoriasis Study of Health Outcomes, PSoHO), an ongoing 3-year observational cohort study in adults with chronic moderate-to-severe plaque psoriasis (sample size of 1981 patients), highlighted the short-term efficacy of IL-17A inhibitors, in particular ixekizumab (Citation23). Furthermore, a literature review concluded that real-world use of ixekizumab in patients with psoriasis is effective and safe, with high treatment persistence and adherence (Citation24).
Improvement of quality of life in patients affected by psoriasis is another important aspect, and it has been previously established that PASI and DLQI correlate in patients with moderate-to-severe plaque psoriasis (Citation25–27). Treatment with ixekizumab was associated with an improvement of quality of life, and the average DLQI score at the end of study indicated that their psoriasis condition had ‘minimal effect on patients’ lives.’ In addition, among patients with DLQI 0 or 1 at Month 24, most of them showed complete skin clearance (PASI 100), suggesting that maintaining PASI 100 over a long period of time may have an even greater impact on quality of life in patients with psoriasis. This is consistent with the results of the long-term extension of the UNCOVER trials, which indicated that among patients with PASI 100 responses, 92% achieved DLQI (0, 1) indicating no impact of skin disease on quality of life (Citation16).
As biologic options for patients with moderate-to-severe plaque psoriasis are becoming increasingly more prevalent, and it has been shown that previous exposure to biologics may have an impact on the effectiveness of treatments, we were interested to see if previous exposure to biologics on patients had any effect on response to ixekizumab. Based on our study results, we determined that previous exposure does not have an evident effect on symptoms improvement of psoriasis, as both subgroups of patients showed similar PASI 75, PASI 90, and PASI 100 response rates at all time points, and the proportions of patients achieving the targeted PASI scores were also similar between the two groups (136 and 62 patients were biologic naïve and biologic experienced, respectively). This is concurrent and consistent with the data from a retrospective Italian real-life study (Citation20), which also concluded that no significant impact of previous exposure to biologics on average PASI improvement was observed. On the contrary, the results from the PSoHO study indicated that patients treated with anti-IL-17A including ixekizumab who were biologic-naïve were able to show higher PASI 90 and PASI 100 response rates compared to patients who were biologic-experienced (Citation28). This finding may be attributed to the different patient populations involved in these studies, which suggests that, in a responder enriched population, patients can benefit from long-term treatment of ixekizumab with the same magnitude regardless of previous exposure to biologics, but for an overall population in real-world setting, patients who are biologic-naïve may have a higher likelihood of experiencing a greater benefit from ixekizumab use (Citation28).
Our study reported an overall high ixekizumab drug retention, with only 4% of discontinuation rate. This is consistent with the results from Malagoli et al. (Citation20), in which the treatment suspension rate was 6%. A recent real-world study also demonstrated that ixekizumab had a better drug survival over two years compared to other biologics in patients with psoriasis (Citation29). As it has been previously reported that adherence rates in patients with psoriasis, regardless of treatment types, are suboptimal (Citation8), there is a need to improve patient compliance in this population. Our result suggests that a low discontinuation rate of ixekizumab would be beneficial in the psoriasis population, in which patient compliance is typically less than optimal. Albeit we did not observe a difference in the discontinuation rate between biologic-naïve and biologic-experienced patients, previous studies investigating drug survival of biologic agents used for the treatment of psoriasis identified the prior exposure to biologic agents as one of the predictors for drug discontinuation (Citation20,Citation30,Citation31).
The main limitation of this study is that the analysis has been performed on a responder enriched population of patients who were treated with ixekizumab for at least 12 months. This could have easily excluded patients who did not respond to ixekizumab, and consequently overestimated the response rates and improvement in plaque psoriasis, as well as ixekizumab drug retention. Due to the observational and retrospective nature, other limitations of the study include missing data and lack of internal validity, incomplete data, difficulty in interpreting or verifying documented information, and variability between patients in the quality of documentation.
Supplemental Material
Download PDF (89.9 KB)Acknowledgements
The authors would like to thank the participants for their participation and cooperation. The authors would also like to thank OPIS s.r.l. for conducting the study on behalf of the sponsor. Manuscript writing assistance in the preparation of this article was provided by Chu-Han Huang from OPIS s.r.l.
Disclosure statement
A. Chiricozzi has served as advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Eli Lilly. A. Balato has served as board participant or speaker for Eli-Lilly. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Eli Lilly. A. Giunta served as consultant, board member and speaker for and/or received research grants from Eli Lilly. P. Dapavo has acted as a speaker or consultant for Eli Lilly. G. Carrera has served as a board participant or speaker for Eli Lilly. A. Parodi and S. Mazzoccoli have reported conflicts of interests with Eli Lilly. C. Buzzoni and S. Sabatino are employed and minor shareholders at Eli Lilly. No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, C.B., upon reasonable request.
Additional information
Funding
References
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1–6. doi: 10.1001/jama.2020.4006.
- Parisi R, Symmons DP, Griffiths CE, Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) Project Team, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339.
- Pezzolo E, Cazzaniga S, Colombo P, et al. Psoriasis incidence and lifetime prevalence: suggestion for a higher mortality rate in older age-classes among psoriatic patients compared to the general population in Italy. Acta Derm Venereol. 2019;99(4):400–403. doi: 10.2340/00015555-3130.
- Di Meglio P, Villanova F, Nestle FO. Psoriasis. Cold Spring Harb Perspect Med. 2014;4(8):a015354. doi: 10.1101/cshperspect.a015354.
- Carvalho AV, Romiti R, Souza CD, et al. Psoriasis comorbidities: complications and benefits of immunobiological treatment. An Bras Dermatol. 2016;91(6):781–789. doi: 10.1590/abd1806-4841.20165080.
- Kolli SS, Amin SD, Pona A, et al. Psychosocial impact of psoriasis: a review for dermatology residents. Cutis. 2018;102(5S):21–25.
- Armstrong AW, Puig L, Joshi A, et al. Comparison of biologics and oral treatments for plaque psoriasis: a meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi: 10.1001/jamadermatol.2019.4029.
- Belinchón I, Rivera R, Blanch C, et al. Adherence, satisfaction and preferences for treatment in patients with psoriasis in the European Union: a systematic review of the literature. Patient Prefer Adherence. 2016;10:2357–2367. doi: 10.2147/PPA.S117006.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. doi: 10.1007/s00403-010-1080-1.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs. An Bras Dermatol. 2017;92(5):668–674. doi: 10.1590/abd1806-4841.20175603.
- Azhar A, Zaayman M, Silfvast-Kaiser A, et al. Ixekizumab in the treatment of moderate-to-severe plaque psoriasis: patient adherence, satisfaction, and preferences. Dermatol Ther. 2021;34(1):e14486. doi: 10.1111/dth.14486.
- Gordon KB, Blauvelt A, Papp KA, UNCOVER-1 Study Group; UNCOVER-2 Study Group; UNCOVER-3 Study Group., et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375(4):345–356. doi: 10.1056/NEJMoa1512711.
- Papp KA, Leonardi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double-blinded, controlled studies (UNCOVER-1, UNCOVER-2, UNCOVER-3). Br J Dermatol. 2018;178(3):674–681. doi: 10.1111/bjd.16050.
- Leonardi CL, Blauvelt A, Sofen HL, et al. Rapid improvements in health-related quality of life and itch with ixekizumab treatment in randomized phase 3 trials: results from UNCOVER-2 and UNCOVER-3. J Eur Acad Dermatol Venereol. 2017;31(9):1483–1490. doi: 10.1111/jdv.14211.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79(5):824–830.e2. doi: 10.1016/j.jaad.2018.05.032.
- Leonardi C, Reich K, Foley P, et al. Efficacy and safety of ixekizumab through 5 years in moderate-to-severe psoriasis: long-term results from the UNCOVER-1 and UNCOVER-2 phase-3 randomized controlled trials. Dermatol Ther. 2020;10(3):431–447. doi: 10.1007/s13555-020-00367-x.
- Blauvelt A, Lebwohl MG, Mabuchi T, et al. Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. 2021;85(2):360–368. doi: 10.1016/j.jaad.2020.11.022.
- Damiani G, Conic RRZ, Pigatto PDM, Young Dermatologists Italian Network, et al. From randomized clinical trials to real life data. An Italian clinical experience with ixekizumab and its management. Dermatol Ther. 2019;32(3):e12886. doi: 10.1111/dth.12886.
- Chiricozzi A, Burlando M, Caldarola G, et al. Ixekizumab effectiveness and safety in the treatment of moderate-to-severe plaque psoriasis: a multicenter, retrospective observational study. Am J Clin Dermatol. 2020;21(3):441–447. doi: 10.1007/s40257-019-00490-2.
- Malagoli P, Dapavo P, Pavia G, et al. Real life long-term efficacy and safety of ixekizumab in moderate-to-severe psoriasis: a 192 weeks multicentric retrospective study-IL PSO (Italian landscape psoriasis). Dermatol Ther. 2022;35(8):e15608. doi: 10.1111/dth.15608.
- Deza G, Notario J, Lopez-Ferrer A, et al. Initial results of ixekizumab efficacy and safety in real-world plaque psoriasis patients: a multicentre retrospective study. J Eur Acad Dermatol Venereol. 2019;33(3):553–559. doi: 10.1111/jdv.15288.
- Gulliver W, Penney M, Power R, et al. Moderate-to-severe plaque psoriasis patients treated with ixekizumab: early real-world outcomes and adverse events. J Dermatolog Treat. 2022;33(1):354–360. doi: 10.1080/09546634.2020.1755009.
- Pinter A, Puig L, Schäkel K, et al. Comparative effectiveness of biologics in clinical practice: week 12 primary outcomes from an international observational psoriasis study of health outcomes (PSoHO). J Eur Acad Dermatol Venereol. 2022;36(11):2087–2100. doi: 10.1111/jdv.18376.
- Reich A, Reed C, Schuster C, et al. Real-world evidence for ixekizumab in the treatment of psoriasis and psoriatic arthritis: literature review 2016–2021. J Dermatolog Treat. 2023;34(1):2160196. doi: 10.1080/09546634.2022.2160196.
- Gerdes S, Körber A, Biermann M, et al. Absolute and relative psoriasis area and severity index (PASI) treatment goals and their association with health-related quality of life. J Dermatolog Treat. 2020;31(5):470–475. doi: 10.1080/09546634.2020.1746734.
- Prevezas C, Katoulis AC, Papadavid E, et al. Short-Term correlation of the psoriasis area severity index, the nail psoriasis area severity index, and the dermatology life quality index, before and after treatment, in patients with skin and nail psoriasis. Skin Appendage Disord. 2019;5(6):344–349. doi: 10.1159/000499348.
- Mattei PL, Corey KC, Kimball AB. Psoriasis area severity index (PASI) and the dermatology life quality index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–337. doi: 10.1111/jdv.12106.
- Lynde C, Riedl E, Maul JT, et al. Comparative effectiveness of biologics across subgroups of patients with moderate-to-severe plaque psoriasis: results at week 12 from the PSoHO study in a real-world setting. Adv Ther. 2023;40(3):869–886. doi: 10.1007/s12325-022-02379-9.
- Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the corona psoriasis registry. Dermatol Ther. 2021;34(2):e14808. doi: 10.1111/dth.14808.
- Caldarola G, Chiricozzi A, Megna M, et al. Real-life experience with ixekizumab in plaque psoriasis: a multi-center, retrospective, 3-year study. Expert Opin Biol Ther. 2023;23(4):365–370. doi: 10.1080/14712598.2023.2193288.
- Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)-17 and IL-23 inhibitors for the treatment of psoriasis: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904. doi: 10.1007/s40257-022-00722-y.

