Abstract
Background
Non-immunosuppressed patients with a history of multiple non-melanoma skin cancers (NMSCs) taking oral nicotinamide supplementation experienced a 23% decrease in annual NMSC risk in a randomized clinical trial. Patient preferences for risks and costs associated with nicotinamide are unknown.
Objectives
To understand how patients prioritize NMSC reduction, infection risk, and cost.
Methods
A sample of adults with history of ≥2 NMSC within the past five years undergoing Mohs procedure completed a discrete-choice experiment comprising two hypothetical treatments—characterized by varying reductions in NMSC incidence, increased severe infection risk, and cost—and no treatment. The data were analyzed with random-parameters logit models.
Results
A total of 203 subjects (mean age 71.5 years, 65.5% males) participated. For a 23% annual reduction in NMSC incidence, a 26% [95% CI: 8%–45%] annual increase in severe infection risk and $8 [95% CI: $2–14] monthly cost was acceptable. Outcomes across analyzed subgroups (before vs. during COVID pandemic, site of interview, less vs. more prior NMSCs) were similar.
Conclusions
Patients were unwilling to accept high severe infection risks to obtain the reduction in NMSC incidence observed in a nicotinamide trial, suggesting that routinely recommending nicotinamide may run counter to some patients’ preferences.
Introduction
As more than 3 million non-melanoma skin cancers (NMSCs) are diagnosed in the United States annually (Citation1), associated with approximately 2000 annual deaths, substantial morbidity, and treatment costs estimated in 2014 at $4.8 billion (Citation2), effective and safe interventions to reduce NMSC incidence are desirable.
Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) was a randomized, placebo-controlled trial in which oral nicotinamide taken by non-immunosuppressed individuals with a history of multiple NMSCs was associated with a reduction in the annual incidence of NMSC (p = .02), with patients randomized to nicotinamide experiencing a 23% greater reduction in NMSC (95% confidence interval [CI]: 4%–38%) than did placebo patients (Citation3). Zhao noted that patients in the nicotinamide arm had higher total infections and severe infections (10/193) compared to placebo (5/193) (Citation4). This difference in severe infection risk was not statistically significant, but the ONTRAC trial was powered for detecting differences in NMSC incidence, not severe infection risk.
Whether patients with a history of multiple NMSCs consider a 23% reduction in NMSC risk sufficient to justify the nuisance, expense, and unknown but possible risks of oral nicotinamide is unknown. This study uses a discrete-choice experiment (DCE) to examine the willingness of such patients to take an oral supplement, accepting costs and adverse events, for a reduced risk of future NMSC.
DCE methodology presupposes that a medical intervention consists of a set of attributes and that the intervention’s attractiveness depends on these attributes. In a DCE, respondents consider different hypothetical treatments and indicate their preferred treatment. The choice reveals their preferences and which tradeoffs they are willing to accept.
Patients and methods
Adult patients with a history of ≥ 2 NMSCs in the past five years undergoing Mohs surgery at four sites in the United States for NMSC at the time of taking the survey were included (respondents were enrolled and asked to complete the survey between stages of their Mohs procedure, when logistically convenient). Informed, written consent was obtained from all patients. Western IRB determined this study was exempt from institutional review board review. Due to the operational constraints of conducting this study in busy surgery clinics, it was not practical to collect information about the response rate or to characterize the non-responders to ensure that nonresponse bias doesn’t threaten study validity. Our assumption was that if subjects were not interested in completing a survey, then it is unlikely that they would be interested in taking a supplement, in which case nonresponse bias likely results in overestimation of willingness to take supplement.
The inclusion and exclusion criteria emulated ONTRAC, so that ONTRAC efficacy and safety results can be inferred to apply. The requirement for patients to be undergoing Mohs surgery ensured participants were acutely aware of NMSC-associated treatment morbidity.
Patients meeting the survey criteria were invited by their surgeon to participate and were provided with detailed written explanations of the meaning and descriptions of the attributes in the pen-and-paper DCE. Participant enrollment was 203 patients.
Table E1 in Supplementary Material summarizes attributes and attribute levels for the DCE questions. Attribute levels were designed to bracket point estimates of NMSC rate reduction and severe infection risk observed in ONTRAC, as well as nicotinamide retail cost (see Supplementary Material). In the questions, attribute levels were expressed in percentage terms for benefit and risk (e.g., 10% reduction in skin cancer, 25% increase in the risk of severe infection). The baseline risk of severe infection was set at 2.6%, which corresponds to the annual risk of severe infection among placebo subjects in ONTRAC.
Each question included two oral supplement options with differing levels of future NMSC reduction, infection risk and cost and a ‘No supplement’ option. Combinations of attribute levels defining the supplement alternatives and the full set of questions in the DCE were determined by an experimental design developed to allow estimation of the main-effect preference weights of interest using a random-parameters logit (RPL) model (Citation5). The commonly employed D-optimal algorithm developed in SAS 9.4 software (SAS Institute Inc, Cary, North Carolina) was used to construct a fractional factorial experimental design (Citation6,Citation7) using the attribute levels presented in Supplementary Material Table E1. The design was evaluated for level balance and correlation. The DCE survey instrument and experimental design were developed following ISPOR (the Professional Society for Health Economics and Outcomes Research) good research practice guidelines (Citation5).
The experiment included 24 DCE questions divided into two blocks of 12 questions. Respondents were randomly assigned to one of the two blocks. The question order in each block was randomized for each respondent to avoid learning and fatigue biases that could contribute to measurement error (Citation8,Citation9).
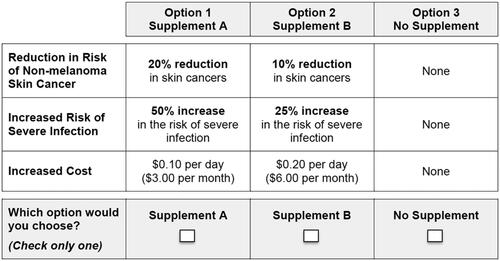
In each question, respondents were asked which of three options they would choose if these were the only options available (a sample choice question is presented in ).
Figure 1. Example of a discrete-choice experiment question. Respondents were asked which of three options they would choose if these were the only options available.
Data from the DCE were analyzed using an RPL model, which mitigates potential estimation bias in the mean preference weight estimates that may occur because of unobserved preference heterogeneity among respondents; the RPL model mitigates this potential bias by estimating a distribution around each mean preference parameter (Citation10,Citation11).
Statistical analysis of DCE data was conducted following research practice guidelines published by ISPOR (Citation12). For the analysis, a main-effects specification of the utility function was adopted (no interactions). Each estimated parameter was assumed normally distributed and tested in model estimation. Multiple model and parameter specifications were tested (Citation13), and the final model specification was selected as discussed in the results section. The delta method (Citation14) was used to calculate standard errors for the preference weight for each omitted attribute level.
Conditional relative importance of attributes was calculated as the difference between the preference weight for the most-preferred and least-preferred level of each attribute. Standard errors and 95% CIs for differences were calculated using the delta method. Conditional relative importance estimates are rescaled such that their sum is equal to 100; therefore, each one can be interpreted as the proportion of utility that can be gained by improving each attribute from the least- to the most-preferred level relative to the maximum utility gained from improving all attributes.
We estimated (1) maximum acceptable risk (MAR) of severe infection for various reductions in NMSC risk and (2) willingness to pay (WTP) for reductions in NMSC risk and risk of severe infection. Preference weights for risk and cost are interpreted as the incremental disutility caused by a 1% increase in risk and a $1 increase in cost for the treatment. The MAR is computed as the negative ratio between the utility gained when increasing the benefit and the disutility of the risk of severe infection, while the WTP is computed as the negative ratio between the increased benefit or reduced risk and the disutility of cost. These can be interpreted as how respondents balance risk, cost, and benefit.
Results
Demographic and clinical history statistics for survey participants matched expectations for a patient population with multiple prior NMSCs (). Since patients with multiple prior NMSCs may have difficulty remembering their exact number of prior NMSCs, they were asked to classify prior NMSCs into prespecified ranges (2, 3–5, 6–9, 10, or more).
Table 1. Descriptive statistics (N = 203).
The ‘No supplement’ option was selected 4464 (62%) times, and 61 respondents (30%) always selected the ‘No supplement’ alternative.
To explore which model best fit the data, we estimated multiple RPL models varying the assumptions regarding how data were coded and how the heterogeneity was incorporated. Initially, all attribute levels were effect coded and the alternative specific constant for the ‘No supplement’ option was dummy coded to avoid confounding effects between the constant and the preference weights. Looking at the preference weight estimates from this initial model, the preferences for risk of severe infection and for cost were best described with a linear and polynomial function, respectively, while efficacy was well described by a categorical specification (effects-coded) (Figure E1 in Supplementary Material). Therefore, the final model specification used a linear function for the risk, a polynomial (square) function for cost and efficacy was effects-coded. In this final model the alternative specific constant was fully captured by the effects-coded variable estimated for zero efficacy. The RPL model was first estimated with all parameters normally distributed. Distribution for cost was not statistically significant; therefore, cost parameters were fixed in the final distribution. All models included an error component to correlate the 2 designed alternatives.
The full model output is presented in the Supplementary Material, Table E2, and plots the mean preference estimate for each attribute (vertical bars represent the 95% CI). Preference weights are relative and do not have an absolute interpretation. Attribute levels with larger preference weights are preferred to attribute levels with smaller preference weights. If the CIs for any pair of levels of the same attribute do not overlap, mean estimates for those attribute levels are statistically significantly different. Preference weights were ordered as expected, with better outcomes being preferred to worse outcomes.
Figure 2. Preference weights, random-parameters logit model estimates, final model specification (N = 203). Attributes are presented in the order in which they appeared in the DCE questions. The vertical bars around each mean preference weight represent the 95% CI around the point estimate. The levels for the reduction in risk of non-melanoma skin cancer are effects-coded, so the sum of preference weights across levels of an attribute equals zero; 50% reduction in skin cancers was omitted in model estimation and retrieved for the plot. Within each attribute, a higher preference weight indicates that a level is more preferred. The change in utility associated with a change in the levels of each attribute is represented by the vertical distance between the preference weights for any two levels of that attribute. Larger differences between preference weights indicate that respondents viewed the change as having a relatively greater effect on overall utility.
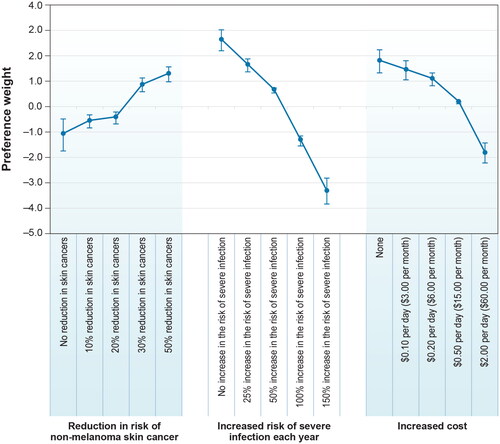
Figure 3. Scaled conditional relative importance of attributes, random-parameters logit model estimates, final model specification (N = 203). Attributes are presented in the order in which they appeared in the DCE questions. The conditional relative importance is the difference between the coefficient on the most- and least-preferred attribute level. These differences are summed across attributes and the sum is scaled to 100. The standard errors and the 95% CI for these differences were calculated using the delta method. The 95% CI around the point estimate is represented by the black vertical bars on top of the blue bars.
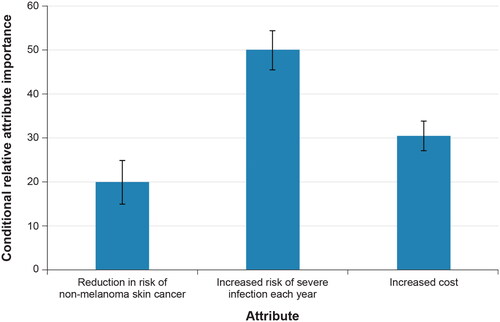
A plot of the conditional relative importance for each attribute demonstrates that increased risk of severe infection was the most important attribute, and reduced risk of NMSC was the least important attribute (). Attributes are presented in the order in which they appeared in the DCE questions. The conditional relative importance is the difference between the coefficient on the most- and least-preferred attribute level. These differences are summed across attributes and the sum is scaled to 100. The standard errors and the 95% CI for these differences were calculated using the delta method. The 95% CI around the point estimate is represented by the black vertical bars on top of the blue bars.
Tables E3 and E4 in the Supplementary Materials show all the MARs of severe infection computed for various reductions in NMSC risk as well as all the WTP computed for both reductions in NMSC risk and reductions in severe infection risk. Both MAR and WTP were estimated by interpolating between the ‘No reduction’ and ‘30% reduction’ levels and between the ‘No reduction’ and ‘20% reduction’ levels, which bracket the NMSC risk reduction observed in ONTRAC (23%). For the nicotinamide-associated NMSC risk reduction, the MAR of severe infection each year is estimated at 26.4% (7.5%–45.2%; 95% CI); the observed increased relative risk of severe infection associated with nicotinamide in ONTRAC was 100%. The WTP for the nicotinamide-associated NMSC risk reduction is estimated at $8.41 per month ($2.45–$14.37; 95% CI); the observed retail cost for nicotinamide ranged from $3 to $14.40 per month at a dose of 500 mg bid (Citation15).
Subgroups were explored based on the following characteristics: (1) before/after the COVID-19 pandemic, (2) site of interview, and (3) lifetime number of NMSCs, with figures presenting preference weights and conditional relative importance for each subgroup included in the Supplementary Materials (Figures E2–E7).
Fifty-four respondents were interviewed before pandemic onset. Willingness to tolerate risk of severe infection was not statistically significantly different (Wald test p = .59) before versus after pandemic onset (Figures E2 and E3 in Supplementary Material).
The four practices that participated in the study differed in the economic status of the populations served. Median household income (2019 inflation-adjusted dollars) for the zip code areas for the sites were: Chevy Chase, MD ($156,686); Austin, TX ($85,555); Hagerstown, MD ($65,638); Fort Atkinson, WI ($61,815) (Citation16). Preferences between the former 2 and the latter 2 sites were not statistically significantly different (Wald test p value = .30) (Figures E4 and E5 in Supplementary Material).
The preferences of patients with a history of ≤ 5 NMSCs versus those with ≥ 6 NMSCs were statistically significantly different (Wald test p value = .03). Respondents with more lifetime NMSCs placed more importance on cost, though differences were small (Figures E6 and E7 in Supplementary Material).
Discussion
This study used a DCE to quantify benefit-risk and benefit-cost tradeoffs among patients with a history of multiple NMSCs for an oral supplement to reduce the risk of future NMSCs. Nicotinamide at a dose of 500 mg bid significantly reduces the risk of NMSC in non-immunosuppressed patients with a history of multiple NMSC (Citation3) and possibly elevates the risk of severe infection (Citation4). For the benefit of reducing risk of future NMSCs gained by nicotinamide supplementation, our respondents were not willing to accept the increased risks of severe infection observed in ONTRAC. They were willing to pay the cost for nicotinamide. Because our criteria excluded immunosuppressed patients, these conclusions cannot be applied to such patients, for whom the NMSC risk is higher and the risk-benefit balance of nicotinamide supplementation appears to differ, given that a placebo-controlled trial of oral nicotinamide in solid-organ transplant recipients failed to demonstrate significant reduction in NMSC risk (Citation17).
ONTRAC was not designed to provide a precise estimate of the increased risk of severe infections associated with nicotinamide. It is plausible that the observed increased risk of severe infections is spurious. ENDIT, a 552-subject randomized double-blind placebo-controlled trial of nicotinamide to prevent type I diabetes, did not demonstrate any differences between the treatment groups in adverse events (though the ENDIT patient population was younger than ONTRAC’s) (Citation18). Also, a recent review on the safety of high-dose nicotinamide did not report any evidence of increased risk of severe infection with supplementation (Citation19). In contrast, the placebo-controlled trial of oral nicotinamide in solid-organ transplant recipients with a history of multiple NMSCs (ONTRANS) also demonstrated a numerically increased risk of severe infections among patients randomized to oral nicotinamide (Citation17), compared to those randomized to placebo. While it may seem counterintuitive to expect an increased risk of severe infections associated with nicotinamide supplementation, a decrease in CD68-positive macrophages has been noted in skin tumors of patients taking nicotinamide (Citation20), and CD68 expression is upregulated in macrophages in response to inflammatory stimuli (Citation21). To rule out definitively an increased risk of severe infection in a population at risk for NMSC, a prospective study would need to be so large as to be impractical. The proposed possible risks of a severe infection, even if spurious, may have been interpreted by patients as a proxy for any serious adverse event, in which case this DCE demonstrates that these patients generally have a low tolerance for risk in exchange for a modest reduction in NMSC rate.
It is plausible that a DCE conducted partially during a global pandemic would elicit elevated concerns about the risk of severe infection. However, when subgroup analysis was performed, the results were similar before and during the pandemic. As this survey was not designed to look for differences between subgroups, conclusions from this and other subgroup analyses should be interpreted cautiously.
This DCE has other important limitations. As in all stated-preference studies, respondents were asked to make hypothetical choices, which may differ from actual decisions made in a clinical setting.
A recent meta-analysis of the effect of nicotinamide in skin cancer chemoprophylaxis identified the Chen et al. study as having low risk of bias (Citation22). However, it is possible that Chen et al. generated an imprecise estimate of nicotinamide efficacy. It is also uncertain whether results from 4 clinical locations can be generalized to the United States population. Additionally, there was a practical limit to how many attributes could be evaluated in this study, given the sample size and the necessity of avoiding survey fatigue, which carries the risk of generating unreliable data. Consequently, only the most important attributes could be included. Many known or theoretical nicotinamide attributes—both beneficial and detrimental—were not evaluated. Nicotinamide-associated diarrhea and flushing may dissuade patients. High nicotinamide levels may stress the mitochondria of dopaminergic neurons, potentially triggering neuronal apoptosis and increasing the risk of Parkinson’s disease (Citation23). Aside from the reduction in NMSC risk, other potential benefits associated with nicotinamide supplementation (e.g., beneficial effects on aging), but not included in the study, might persuade patients to take the supplement (Citation24). Questions about patients’ income were not included in the survey because of concerns it would discourage participation; however, inability to adjust for income levels makes interpreting the cost-benefit tradeoff difficult. Most importantly, patients’ individual risk tolerances vary.
These results suggest that patients with a history of multiple NMSCs have a low tolerance for increased risk of severe infection. According to the current literature, risk of severe infection may be a potential adverse event, so dermatologists recommending nicotinamide for chemoprevention should clearly inform patients about the possible risk. Given the uncertainty of the risk profile, clinicians should be cautious about routinely recommending nicotinamide supplementation to patients with a history of NMSCs.
Author contributions
Marco Boeri: method (lead); data analysis (lead); writing-original draft (supporting); writing-review and editing (supporting). Maral K. Skelsey: data curation (supporting); writing-review and editing (supporting). James A. Schiro: data curation (supporting); writing-review and editing (supporting). Susan E. Dozier: data curation (supporting); writing-review and editing (supporting). Robert Glinert: data curation (supporting); writing-review and editing (supporting). Martin M. Okun: conceptualization (lead); methodology (supporting); data curation (lead); writing-original draft (supporting); writing-review and editing (supporting).
Supplemental Material
Download PDF (1.3 MB)Acknowledgments
We are thankful to the patients who participated at the clinics involved and to Jheeyae Ahn who assisted in collecting surveys and to A. Brett Hauber for helpful discussions during the study design. Martin M. Okun is Consultant for AbbVie, Alumis, Azora Therapeutics, Bluefin Biomedicine, Boehringer Ingelheim, Incyte, Insmed, Novartis, Phoenicis, Regeneron, Vyne Therapeutics. No other authors have conflicts.
Disclosure statement
Martin M. Okun is Consultant for AbbVie, Alumis, Azora Therapeutics, Bluefin Biomedicine, Boehringer Ingelheim, Chemocentryx, Incyte, Insmed, Novartis, Phoenicis, Regeneron, Vyne Therapeutics. No other authors have conflicts.
Additional information
Funding
References
- cancer.net. Skin cancer (non-melanoma): statistics. Available from: https://www.cancer.net/cancer–types/skin–cancer–non–melanoma/statistics
- United States Department of Health and Human Services. The surgeon general’s call to action to prevent skin cancer. Washington (DC): Office of the Surgeon General; 2014.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin–cancer chemoprevention. N Engl J Med. 2015;373(17):1–6. doi:10.1056/NEJMoa1506197.
- Zhao Y. Nicotinamide for skin–cancer chemoprevention. N Engl J Med. 2016;374(8):789. doi:10.1056/NEJMc1514791.
- Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete–choice experiments: report of the ISPOR conjoint analysis discrete–choice experiment experimental design good research practices task force. Value Health. 2013;16:3–13. doi:10.1016/j.jval.2012.08.2223.
- Kuhfeld W. Marketing research methods in SAS: experimental design, choice, conjoint, and graphical techniques. Cary (NC): SAS Institute Inc.; 2010.
- Kuhfeld W, Tobias F, Garratt M. Efficient experimental design with marketing research applications. J Mark Res. 1994;31(4):545–557. doi:10.1177/002224379403100408.
- Campbell D, Boeri M, Doherty E, et al. Learning, fatigue and preference formation in discrete choice experiments. J Econ Behav Organ. 2015;119:345–363. doi:10.1016/j.jebo.2015.08.018.
- Maddala T, Philips K, Johnson F. An experiment simplifying conjoint analysis designs for measuring preferences. Health Econ. 2003;12(12):1035–1047. doi:10.1002/hec.798.
- Train K. Discrete choice methods with simulation. 2nd ed. Cambridge (UK): Cambridge University Press; 2009.
- Train K, Sonnier G. Mixed logit with bounded distributions of correlated partworths. In: Scarpa R, Albertini A, editors. Applications of simulation methods in environmental and resource economics. Dordrecht: Springer Publisher; 2005. p. 117–134.
- Hauber A, González J, Groothuis–Oudshoorn C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis experimental design task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004.
- Gonzalez JM, Boeri M. The impact of the risk functional form assumptions on maximum acceptable risk measures. Patient. 2021;14(6):827–836. doi:10.1007/s40271-021-00518-y.
- Hensher D, Rose J, Greene W. Applied choice analysis. Cambridge (UK): Cambridge University Press; 2005.
- Amazon. 2022. Available from: https://www.amazon.com/Nutricost-Niacinamide-Vitamin-500mg-Capsules/dp/B01M9GIV8S/ref=sr_1_2?keywords=nicotinamide&qid=1664366497&qu=eyJxc2MiOiI0LjQzIiwicXNhIjoiMy45NSIsInFzcCI6IjMuODcifQ%3D%3D&sr=8-2&th=1
- United States Census Data. Available from: www.data.census.gov
- Allen NC, Martin AJ, Snaidr VA, et al. Nicotinamide for skin-cancer chemoprevention in transplant recipients. New Eng J Med. 2023;388(9):804–812. doi:10.1056/NEJMoa2203086.
- Gale EA, Bingley PJ, Emmett CL, et al. European nicotinamide diabetes intervention trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet. 2004;363(9413):925–931. doi:10.1016/S0140-6736(04)15786-3.
- Hwang ES, Song SB. Possible adverse effects of high-dose nicotinamide: mechanisms and safety assessment. Biomolecules. 2020;10(5). doi:10.3390/biom10050687.
- Minocha R, Martin AJ, Chen AC, et al. A reduction in inflammatory macrophages may contribute to skin cancer chemoprevention by nicotinamide. J Invest Dermatol. 2019;139(2):467–469. doi:10.1016/j.jid.2018.08.018.
- Chistiakov DA, Killingsworth MC, Myasoedova VA, et al. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2017;97(1):4–13. doi:10.1038/labinvest.2016.116.
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis. J Cutan Med Surg. 2022;26(3):297–308. doi:10.1177/12034754221078201.
- Williams AC, Ramsden DB. Nicotinamide homeostasis: a xenobiotic pathway that is key to development and degenerative diseases. Med Hypotheses. 2005;65(2):353–362. doi:10.1016/j.mehy.2005.01.042.
- Hong W, Mo F, Zhang Z, et al. Nicotinamide mononucleotide: a promising molecule for therapy of diverse diseases by targeting NAD + metabolism. Front Cell Dev Biol. 2020;8:246. doi:10.3389/fcell.2020.00246.

