Abstract
Purpose
We aimed to systematically evaluate the efficacy and safety of adalimumab biosimilar agents in the treatment of moderate-to-severe plaque psoriasis, in order to provide evidence-based reference data for clinical medicine.
Materials and Methods
Five databases were searched by electronic retrieval: PubMed, Embase, Cochrane Library, WanFang and CNKI (China National Knowledge Internet). The retrieval period was from the establishment of each database up to April 2022. Randomized controlled trials (RCTs) on adalimumab biosimilar agents compared with their reference agents in the treatment of moderate-to-serve plague psoriasis were included. A meta-analysis using RevMan software was applied to 8 RCTs involving 2589 patients.
Results
After 16 weeks of medication, there was no significant difference in the response rates of adalimumab biosimilar agents and their reference agents defined as a decrease in the Psoriasis Area and Severity Index (PASI) of ≥75% (PASI 75) (p > 0.05), or in the PASI 50, PASI 90 and PASI 100 measures (p > 0.05). After 16 weeks and 24 weeks of medication, there was no significant difference in the incidence rate of serious adverse events (SAEs) between adalimumab biosimilar agents and their reference agents (p > 0.05). After 16 weeks, 24 weeks and 51 weeks of medication, there was no significant difference in withdrawal rate due to SAEs, treatment-emergent adverse events and adverse events of special interest between adalimumab biosimilar agents and their reference agents (p > 0.05).
Conclusion
These findings suggest that biosimilar agents of adalimumab have an overall efficacy and safety profile for psoriasis comparable to those of their reference agents.
Introduction
Psoriasis is an immune-mediated inflammatory disease that has a long time course with frequent relapse, which can affect the patient’s appearance, mental health and quality of life (Citation1,Citation2). Typical clinical manifestations are scaly erythema or plaques that are localized or widespread. The prevalence of psoriasis varies significantly around the world. In the United States, the prevalence of psoriasis ranges from 2% to 4% (Citation3). Meanwhile, in Western Europe, the condition is prevalent in 1.92% of the population, and in high-income Southern Latin American countries, the rate is 1.10% (Citation4). A survey conducted in 2008 found that the prevalence rate in six cities in China was 0.47% (Citation5). The purpose of psoriatic treatment is to control clinical symptoms and gradually improve the quality of life of affected patients (Citation5). The long-term administration of traditional medicines (Citation5–9) such as Acitretin, Methotrexate, Cyclosporine, Glucocorticoids, Azathioprine, and Leflunomide is restricted in due to inadequate efficacy and/or multiple potential toxic side effects (Citation10).
Targeted therapies against tumor necrosis factor (TNF)-α have demonstrated significant clinical benefits for patients with immune-mediated inflammatory diseases (Citation11). Adalimumab, a fully human IgG1 monoclonal antibody, exhibits high affinity and specificity for binding TNF-a (Citation12), and has been shown to be safe and effective in treating both arthritic psoriasis and moderate-to-severe psoriasis in multiple clinical trials (Citation13–18). Adalimumab was first marketed in the US in 2003, and was approved by the US Food and Drug Administration for the treatment of psoriasis in 2008. It was listed in China in 2010. In 2017, China’s State Food and Drug Administration approved its use for treating moderate-to-severe psoriasis.
Once the patent protection of the adalimumab-antigen drug Humira® expired (Citation19), many pharmaceutical companies performed research to develop its biosimilars. There are now various adalimumab biosimilars on the market in China and other countries, such as HLX 03, AVT 02, BI 695501, MSB 11022, ABP 501, BCD-057 and GP 2017, which are significantly cheaper and more accessible than adalimumab (Citation20). However, it is vital to know if the efficacy and safety of adalimumab biosimilar agents differ from those of their reference agents in the treatment of psoriasis. Based on this, the present study performed a meta-analysis to evaluate differences in curative effect and safety between adalimumab and its biosimilars in order to provide evidence-based information for psoriasis medical treatment.
Materials and methods
Eligibility criteria
We conducted a systematic review of primary research literature that included original randomized controlled trials (RCTs) reported on in full-text English-language publications. Our population of interest comprised patients older than 18 years who had moderate-to-severe plaque psoriasis, with an affected body surface area of ≥10%, a Psoriasis Area and Severity Index (PASI) of ≥12 points, and a static Physician Global Assessment (PGA) or Investigator’s Global Assessment (IGA) of at least 3ponits. The enrolled patients were divided into two treatment stages according to the intervention plan and intervention time. In stage 1, the patients were randomly divided into two groups: the test group received an adalimumab biosimilar, and the control group received an adalimumab brand-name drug. Patients received an initial loading dose of 80 mg of the applicable study drug in week 1, followed by 40 mg every other week until week 16. In stage 2, patients who had completed the 16-week treatment in stage 1 entered stage 2 according to certain conditions or unconditionally, and continued on the treatment until the treatment cycle was finished. Some of the subjects in the second-stage cohort continued to use the first-stage intervention (i.e. administration of 40 mg of the adalimumab brand-name drug or the adalimumab biosimilar every other week).
Outcome indicators
Outcomes included efficacy indicators (designated as ①–④) and safety indicators (designated as ⑤∼⑪), defined as follows: ①, the proportion of patients with a decrease of ≥50% in the PASI from baseline (PASI 50); ②, the proportion of patients with a decrease of ≥75% in the PASI from baseline (PASI 75); ③, the proportion of patients with a decrease of ≥90% in the PASI from baseline (PASI 90); ④, the proportion of patients with a decrease of ≥100% in the PASI from baseline (PASI 100); ⑤, occurrence of a serious adverse event (SAE); ⑥, withdrawal rate due to adverse events (AEs) leading to study discontinuation; ⑦, occurrence of infection; ⑧, occurrence of serious infection; ⑨, occurrence of nasopharyngitis; ⑩, occurrence of treatment-emergent adverse events (TEAEs); and ⑪, occurrence of adverse events of special interest (AESIs) such as drug-induced liver damage and allergic reactions.
Search strategy and data sources
Eligible studies were identified by searching for relevant articles published in the following five databases: PubMed, Embase, Cochrane Library, CNKI (China National Knowledge Internet) and WanFang. The retrieval period was from the establishment of each database up to April 2022. A search method combining subject words, keywords and free-text words was adopted, with adjustments made according to the specific databases. The search terms were “adalimumab,” “humira,” “biosimilar,” “psoriasis” and “plaque psoriasis,” along with their Chinese equivalents.
Study selection
According to the inclusion criteria, two researchers independently read the titles and Abstracts for preliminary screening, and then read the full text of those articles that may have met the inclusion criteria. Disagreements were resolved through discussion or consultation. The main extracted data were the first author and publication year, literature source, number of subjects, age, intervention measures, medication treatment, follow-up time, baseline conditions and outcome measures.
Bias and quality evaluations of the literature
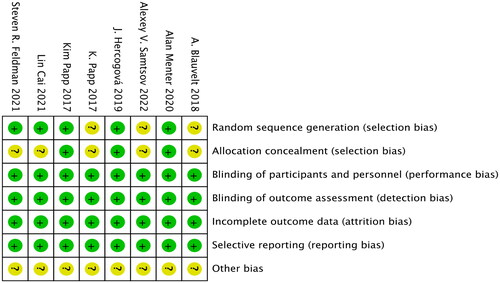
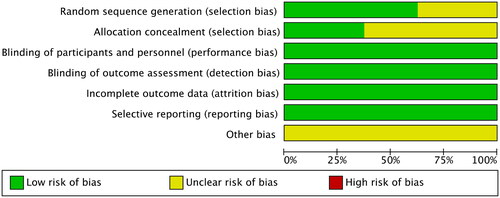
RevMan (version 5.4) software was used to assess the risks of the following seven types of literature bias: generation of random sequences (selection bias), allocation concealment (selection bias), blinding of subjects and investigators (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting of research findings (reporting bias) and other types of bias. The evaluation results are shown in .
The Jadad scale was used to evaluate the quality of the included literature. This scale has a full score of 5 points, and is scored as follows: A score of 1 point is assigned if the trial employed randomization and double-blindness, but the generation of random sequences or the conditions for double-blindness were not specified. A score of 1 point is added if the generation of random sequences or the blinding method is described, and the method is adequate, and the participants have an equal chance of being assigned to the different groups and what the next intervention will be cannot be predicted. A score of 0 points is assigned if a random sequence is generated or the blinding method is unreasonable. A score of 1 point is assigned if the report describes withdrawal or loss to follow-up, and states the number and reasons of withdrawal or loss to follow-up, with a score of 0 points assigned if there is no such description.
Evaluation scores on the Jadad scale of ≥3 are considered to indicate high-quality RCTs (Citation21), while those with scores ≤2 are considered low-quality RCTs. The literature reports included in this study scored ≥3 points on the Jadad scale; the details are presented in .
Table 1. Quality assessment of the included studies.
Statistical analyses
If a study included more than two original studies that evaluated the outcome indicators, statistical analyses were performed using RevMan (version 5.4) software. Accompanied by 95% confidence intervals (CIs), continuous outcomes were pooled for the calculation of weighted mean differences, while categorical outcomes were pooled for the calculation of relative risks (RRs). Study heterogeneity was assessed using the I2 statistic. The cutoff level was α = 0.1, and this was combined with the I2 value in the assessment. Values of I2 ≤ 50% and p > 0.1 were assumed to indicate the absence of between-study statistics heterogeneity, and so a fixed-effects model was used; otherwise, a random-effects model was used due to the assumption that there was heterogeneity between the studies.
Results
Searching and filtering results
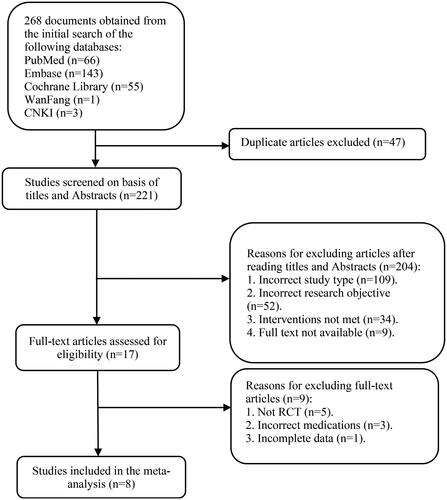
The initial search identified 268 literature reports, of which 47 records were excluded as duplicates, 204 were excluded due to their titles or Abstracts, and 9 articles were excluded after performing full-text screening, which led to 8 articles remaining (Citation22–29). A flow chart of the literature screening process is shown in .
General characteristics of the included literature
The 8 articles involved 2589 patients, with 1295 cases in the experimental group and 1294 cases in the control group. The basic characteristics of each study are presented in . Two studies (Citation26,Citation27) are actually the phases 1 and 2 of the same RCT trial. The eight literature reports covered seven trials. All trials were divided into stage 1 (1–16 weeks) and stage 2 (from the end of week 16 to the end of the trial). One of the studies did not divide the trial into stages (Citation22), and so we divided it into stages 1 and 2 based on the follow-up time. In one of the studies, the same intervention was applied in phases 1 and 2[Citation24). After the completion of stage 1, the two groups of subjects were screened and continued to enter stage 2. In five studies there were various interventions applied in stages 1 and 2 (Citation23,Citation25–29), but both contained a group using biosimilars in phase 1 who directly entered phase 2 or were entered after screening, and the patients using the original drug in stage 1 were re-randomized into two groups to enter phase 2, one of whom continued to be injected with the brand-name drug. It should be noted that phase 2 of the study started at week 25 (Citation28). The details of the studies are presented in .
Table 2. Characteristics of included studies.
Efficacy of adalimumab biosimilars in the treatment of psoriasis
The eight included publications all compared the efficacy indicators between adalimumab biosimilar agents and their reference agents. In six of the studies the subjects had the same intervention (subcutaneous injection of 80 mg of a biosimilar or brand-name drug in week 1 (Citation22,Citation24–26,Citation28,Citation29), followed by the subcutaneous injection of 40 mg of a biosimilar or brand-name drug every other week) and indicator detection time (all patients were assessed using PASI 50, PASI 75, PASI 90 or PASI 100 in week 16), and they were divided into four subgroups according to different efficacy indicators for the meta-analysis described below.
Efficacy when taking medication for 16 weeks
PASI 50 values were compared in two studies (Citation24,Citation26), in which there was no statistical heterogeneity (p = 0.37, I2=0%). The fixed-effects model analysis indicated that there were no statistically significant differences in PASI 50 between the adalimumab biosimilars and their reference agents (RR = 1.00, 95% CI = 0.96–1.05, p = 1.00; ).
Figure 4. Forest plots of PASI 50, PASI 75, PASI 90 and PASI 100 values for adalimumab use in patients with psoriasis.
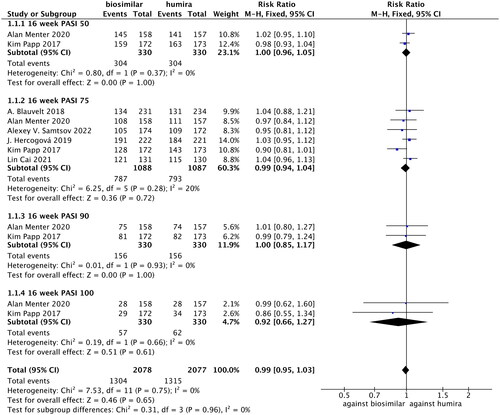
PASI 75 values were compared in six studies (Citation22,Citation24–26,Citation28,Citation29), in which there was no statistical heterogeneity (p = 0.28, I2=20%). The fixed-effects model analysis indicated that there were no significant differences in PASI 75 between the two groups (RR = 0.99, 95% CI = 0.94–1.04, p = 0.72; ).
PASI 90 values were compared in two studies (Citation24,Citation26), in which there was no statistical heterogeneity (p = 0.93, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in PASI 90 between adalimumab biosimilars and their reference agents (RR = 1.00, 95% CI = 0.85–1.17, p = 1.00; ).
PASI 100 values were compared in two studies (Citation24,Citation26), in which there was no statistical heterogeneity (p = 0.66, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in PASI 100 between the two groups (RR = 0.92, 95% CI = 0.66–1.27, p = 0.61; ).
A meta-analysis performed on PASI 50, PASI 75, PASI 90 and PASI 100 found no significant differences in these indicators between the adalimumab biosimilars and their reference agents (RR = 0.99, 95% CI = 0.95–1.03, p = 0.65; ).
Efficacy rates when taking medication for 24, 32 and 48 weeks
One of the studies compared the PASI 75 values for the 20-week, 32-week and 48-week medications (observed in the 50th week) (Citation22), which revealed that PASI 75 values for the biosimilar group and the original research group were 92.7% and 92.3%, respectively, at 20 weeks, 97.2% and 94.7% at 32 weeks, and 96.0% and 93.0% at 48 weeks (all p > 0.05). Another study compared the PASI 75 values for 24 weeks of medication (Citation24), and found that there were 75.3% and 72.4% for the biosimilar drug group and the brand-name drug group, respectively (p > 0.05).
Safety indicators of adalimumab biosimilar agents in the treatment of psoriasis
Safety of taking the medication for 16 weeks
SAEs were compared in three studies (Citation23,Citation26,Citation29), in which there was no statistical heterogeneity (p = 0.21, I2=36%). The fixed-effects model analysis indicated that there were no significant differences in SAE between the adalimumab biosimilar agents and their reference agents (RR = 0.73, 95% CI = 0.36–1.47, p = 0.37; ).
Figure 5. Forest plots of safety indicators for 16 weeks of adalimumab use in patients with psoriasis.
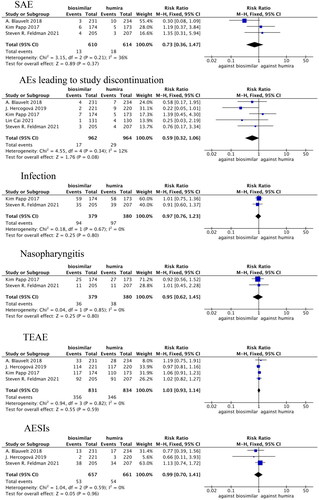
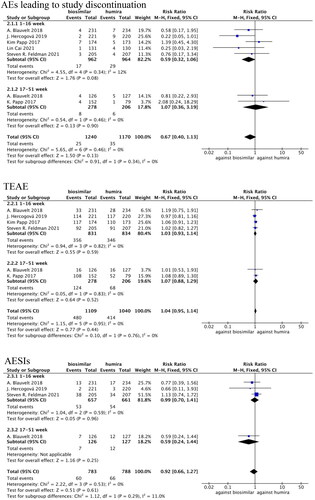
Withdrawal rates due to AEs were compared in five studies (Citation22,Citation23,Citation25,Citation26,Citation29), in which there was no statistical heterogeneity (p = 0.34, I2=12%). The fixed-effects model analysis indicated that there were no significant differences in withdrawal rate due to AEs between the adalimumab biosimilar agents and their reference agents (RR = 0.59, 95% CI = 0.32–1.06, p = 0.08; ).
Infection incidence rates were compared in two studies (Citation23,Citation26), in which there was no statistical heterogeneity (p = 0.67, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in the occurrence of infection between the adalimumab biosimilars and their reference agents (RR = 0.97, 95% CI = 0.76–1.23, p = 0.80; ).
Nasopharyngitis incidence rates were compared in two studies (Citation23,Citation26), in which there was no statistical heterogeneity (p = 0.85, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in the occurrence of nasopharyngitis between adalimumab biosimilar agents and their reference agents (RR = 0.95, 95% CI = 0.62–1.45, p = 0.80; ).
TEAEs were compared in four studies (Citation23,Citation25,Citation26,Citation29), in which there was no statistical heterogeneity (p = 0.82, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in TEAEs between adalimumab biosimilar agents and their reference agents (RR = 1.03, 95% CI = 0.93–1.14, p = 0.59; ).
AESIs were compared in three studies (Citation23,Citation25,Citation29), in which there was no statistical heterogeneity (p = 0.59, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in AESIs between adalimumab biosimilar agents and their reference agents (RR = 0.99, 95% CI = 0.70–1.41, p = 0.96; ).
Safety of taking the medication for 24 weeks
SAEs were compared in two studies (Citation24,Citation28), in which there was no statistical heterogeneity (p = 0.92, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in SAE between the adalimumab biosimilar agents and their reference agents (RR = 0.68, 95% CI = 0.32–1.45, p = 0.32; ).
Figure 6. Forest plots of safety indicators for 1–24 weeks of adalimumab use in patients with psoriasis.
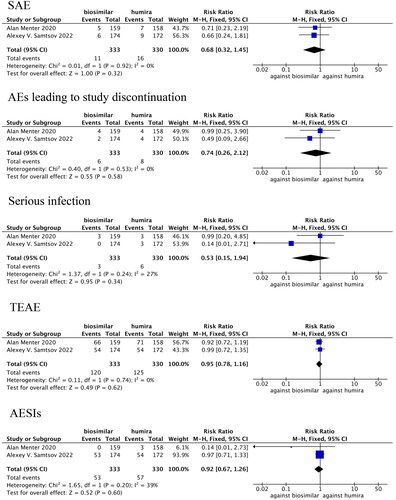
The withdrawal rates due to AEs were compared in two studies (Citation24,Citation28), in which there was no statistical heterogeneity (p = 0.53, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in the withdrawal rate due to AEs between the adalimumab biosimilar agents and their reference agents (RR = 0.74, 95% CI = 0.26–2.12, p = 0.58; ).
The incidence rates of serious infections were compared in two studies (Citation24,Citation28), in which there was no statistical heterogeneity (p = 0.24, I2=27%). The fixed-effects model analysis indicated that there were no significant differences in the incidence of serious infection between the adalimumab biosimilar agents and their reference agents (RR = 0.53, 95% CI = 0.15–1.94, p = 0.34; ).
TEAEs were compared in two studies (Citation24,Citation28), in which there was no statistical heterogeneity (p = 0.74, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in TEAEs between the adalimumab biosimilar agents and their reference agents (RR = 0.95, 95% CI = 0.78–1.16, p = 0.62; ).
AESIs were compared in two studies (Citation24,Citation28), in which there was no statistical heterogeneity (p = 0.20, I2=39%). The fixed-effects model analysis indicated that there were no significant differences in AESIs between the adalimumab biosimilar agents and their reference agents (RR = 0.92, 95% CI = 0.67–1.26, p = 0.60; ).
Safety indicators of taking the medication for 51 weeks
Five studies evaluated three safety indicators at 1–16 weeks (Citation22,Citation23,Citation25,Citation26,Citation29) (the withdrawal rate due to AEs, TEAEs and AESIs) and two studies evaluated the same indicators at 17–51 weeks (Citation27,Citation29). These three indicators were divided into two subgroups according to the medication treatment duration: 1–16 weeks and 17–51 weeks. A meta-analysis was performed and then the results were combined ().
For the withdrawal rate due to AEs, a high degree of homogeneity was seen among the two subgroups, with no statistical heterogeneity among the five studies for 1–16 weeks of medication (p = 0.34, I2=12%). There was also no statistical heterogeneity between the two studies involving 17–51 weeks of treatment (p = 0.46, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in withdrawal rate due to AEs between the adalimumab biosimilar agents and their reference agents at 1–51 weeks (RR = 0.67, 95% CI = 0.40–1.13, p = 0.0.13; ).
For TEAEs there was a high degree of homogeneity in the two subgroups, with no statistical heterogeneity among the four studies involving 1–16 weeks of medication (p = 0.82, I2=0%), or among the two studies involving 17–51 weeks of medication (p = 0.83, I2=0%). The fixed-effects model analysis indicated that there were no significant differences between TEAEs at 1–51 weeks between adalimumab biosimilar agents and their reference agents (RR = 1.04, 95% CI = 0.95–1.14, p = 0.44; ).
For AESIs there was also a high degree of homogeneity in the two subgroups, with no statistical heterogeneity among the three studies involving 1–16 weeks of medication (p = 0.59, I2=0%). The fixed-effects model analysis indicated that there were no significant differences in AESIs at 1–51 weeks between adalimumab biosimilar agents and their reference agents (RR = 0.92, 95% CI = 0.66–1.27, p = 0.61; ).
Sensitivity analysis
A sensitivity analysis was carried out by removing each test index one at a time and changing the effect model. The results showed that these changes did not affect the obtained results, indicating the good stability of the study findings.
Risk-of-bias assessment
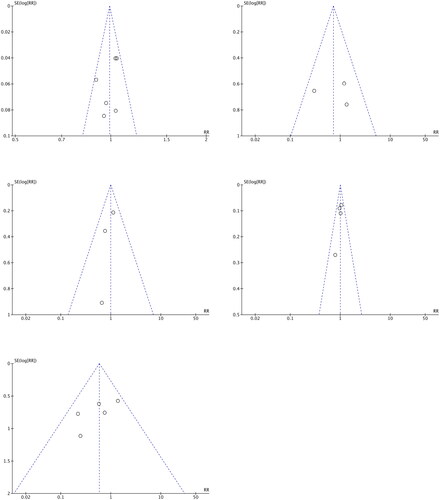
Funnel plots of outcome indicators
RevMan (version 5.4) software was used to draw funnel plots to determine whether publication bias was present for the outcome indicators when more than two studies were included. Funnel plots for the following five indicators are shown in : PASI 75 at 1–16 weeks (Citation22,Citation24–26,Citation28,Citation29), SAE at 1–16 weeks (Citation23,Citation26,Citation29), withdrawal rate due to AEs at 1–16 weeks (Citation22,Citation23,Citation25,Citation26,Citation29), TEAEs at 1–16 weeks (Citation23,Citation25,Citation26,Citation29) and AESIs at 1–16 weeks (Citation23,Citation25,Citation29).
Symmetry tests of the funnel plots
The symmetry of each funnel plot in was assessed using Stata/SE (version 16.0) software to conduct Begg’s rank correlation tests and Egger’s linear regression tests. A probability value larger than 0.05 in these tests indicates that the funnel plot is symmetrical and that there is no publication bias.
Visual inspections of the funnel plots for PASI 75 values at 1–16 weeks, SAEs at 1–16 weeks, withdrawal rates due to AEs at 1–16 weeks, TEAEs at 1–16 weeks and AESIs at 1–16 weeks demonstrated no asymmetry, and there was no publication bias as assessed by Begg’s tests (p = 0.260, 1.000, 0.221, 0.734 and 1.000, respectively) and Egger’s tests (p = 0.312, 0.927, 0.175, 0.496 and 0.411, respectively).
Discussion
This study analyzed seven RCTs, in which the interventions were consistent in stage 1 and partly consistent in phase 2, which enabled us to synthesize and compare them. However, the cycle times of their clinical trials were slightly different: 24 weeks for BI 695501; 48 weeks for HLX 03, AVT 02 and ABP 501; and 51 weeks for MSB 11022, BCD-057 and GP 2017. Therefore, this study performed careful screening during the data extraction and analysis processes, and merged the data obtained under the same conditions. A meta-analysis of the efficacy and safety of adalimumab biosimilar agents and their reference agents was also conducted. The results indicated that the efficacy (PASI 50, PASI 75, PASI 90 and PASI 100) and safety (SAE, nasopharyngitis occurrence, infection occurrence, withdrawal rate due to AEs, TEAEs, AESIs and occurrence of serious infections) of biosimilars (HLX 03, AVT 02, BI 695501, MSB 11022, ABP 501, BCD-057 and GP 2017) and brand-name drugs were very similar. Adalimumab biosimilar agents are cheaper than their reference agents, which therefore makes them good choices for patients.
Our study was subject to some limitations. First, the number of included studies was small, as was the number of included patients. More multicenter, large-sample trials are therefore needed in the future. Second, the inadequate data available in the literature hinder safety and efficacy evaluations of adalimumab biosimilar agents and their reference agents. Third, the studies had a short analysis period of 48–52 weeks, which may not be long enough to capture the complete disease cycle of psoriasis. Psoriasis is a complex, chronic, relapsing, inflammatory, and systemic disease that involves a combination of genetic and environmental factors. As a result, the short test period used in this study may not accurately represent the long-term effects of treatment. Finally, when conducting bias analysis in this article, the results of bias analysis for certain indicators, such as SAE at 1–16 weeks and TEAEs at 1–16 weeks, have limited reference value due to their small amounts of literature included.
Conclusions
Adalimumab biosimilar agents exhibit efficacy and safety profiles that are equivalent to those of their reference agents. These results support adalimumab biosimilar agents as an effective and affordable option for patients with moderate-to-severe plaque psoriasis.
Authors’ contributions
Hui Zhou searched the literature. Yixuan Sunhe extracted the data and performed the statistical analyses. Changkun Li drafted the manuscript. Weihua Dong designed the study and participated in every part of the study process. All of the authors have approved the published version of the manuscript, and agree to be accountable for its accuracy and integrity.
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are stored in the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China), and available from the corresponding author on reasonable request.
Additional information
Funding
References
- Torales J, Echeverría C, Barrios I, et al. Psychodermatological mechanisms of psoriasis. Dermatol Ther. 2020;33(6):1.
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–14. doi: 10.1016/S0140-6736(20)32549-6.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US medicare population: prevalence, treatment, and factors associated with biologic use. J Invest. 2015;135(12):2955–2963. doi: 10.1038/jid.2015.296.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590.
- Committee on Psoriasis CSoD. Guideline for the diagnosis and treatment of psoriasis in China (2018 simplified edition). Chin J Dermatol. 2019;52(4):223–230.
- Nast A, Amelunxen L, Augustin M, et al. S3 guideline for the treatment of psoriasis vulgaris, update - Short version part 1 - Systemic treatment. J Dtsch Dermatol Ges. 2018;16(5):645–669. doi: 10.1111/ddg.13516.
- Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019;71(1):5–32. doi: 10.1002/art.40726.
- Zweegers J, de Jong EM, Nijsten TE, et al. Summary of the Dutch S3-guidelines on the treatment of psoriasis 2011. IJIJDVL. 2014;20(3):21769.
- Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris – update 2015 – short version – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. doi: 10.1111/jdv.13354.
- Christophers E, Griffiths CEM, Gaitanis G, et al. The unmet treatment need for moderate to severe psoriasis: results of a survey and chart review. J Eur Acad Dermatol Venereol. 2006;20(8):921–925.
- Feldmann M, Maini RN. Anti-TNF therapy, from rationale to standard of care: what lessons has it taught us? J Immunol. 2010;185(2):791–794. doi: 10.4049/jimmunol.1090051.
- Kaymakcalan Z, Sakorafas P, Bose S, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009;131(2):308–316. doi: 10.1016/j.clim.2009.01.002.
- Gladman DD, Mease PJ, Ritchlin CT, et al. Adalimumab for long-term treatment of psoriatic arthritis: forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trial. Arthritis Rheum. 2007;56(2):476–488. doi: 10.1002/art.22379.
- Mease PJ, Gladman DD, Ritchlin CT, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(10):3279–3289. doi: 10.1002/art.21306.
- Gordon K, Papp K, Poulin Y, et al. Long-term efficacy and safety of adalimumab in patients with moderate to severe psoriasis treated continuously over 3 years: results from an open-label extension study for patients from REVEAL. J Am Acad Dermatol. 2012;66(2):241–251. doi: 10.1016/j.jaad.2010.12.005.
- Mease PJ, Ory P, Sharp JT, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the adalimumab effectiveness in psoriatic arthritis trial (ADEPT). Ann Rheum Dis. 2009;68(5):702–709. doi: 10.1136/ard.2008.092767.
- Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in Chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi: 10.1111/jdv.13746.
- Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390(10089):40–49. doi: 10.1016/S0140-6736(17)31189-3.
- Sanjeev K, Pankai S, Rajalaxmi N. Opportunities and challenges in biosimilar development Boston: BioProcess International; 2017 [cited 2018]. Available from: http://www.bioprocessintl.com/manufacturing/biosimilars/opportunities-challenges-biosimilar-development/.
- Kim H, Alten R, Avedano L, et al. The future of biosimilars: maximizing benefits across immune-mediated inflammatory diseases. Drugs. 2020;80(2):99–113. doi: 10.1007/s40265-020-01256-5.
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4.
- Cai L, Li LF, Cheng H, et al. Efficacy and safety of HLX03, an adalimumab biosimilar, in patients with moderate-to-severe plaque psoriasis: a randomized, double-blind, phase III study. Adv Ther. 2022;39(1):583–597. doi: 10.1007/s12325-021-01899-0.
- Feldman SR, Reznichenko N, Pulka G, et al. Efficacy, safety and immunogenicity of AVT02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, double-blind, randomised, parallel group, active control, phase III study.BioDrugs. 2021;35(6):735–748. doi: 10.1007/s40259-021-00502-w.
- Menter A, Arenberger P, Balser S, et al. Similar efficacy, safety, and immunogenicity of the biosimilar BI 695501 and adalimumab reference product in patients with moderate-to-severe chronic plaque psoriasis: results from the randomized phase III VOLTAIRE-PSO study. Expert Opin Biol Ther. 2021;21(1):87–96. doi: 10.1080/14712598.2021.1851362.
- Hercogova J, Papp KA, Chyrok V, et al. AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2020;182(2):316–326. doi: 10.1111/bjd.18220.
- Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–1102. doi: 10.1016/j.jaad.2016.12.014.
- Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of the biosimilar ABP 501 compared with adalimumab after single transition: long-term results from a randomized controlled, double-blind, 52-week, phase III trial in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;177(6):1562–1574. doi: 10.1111/bjd.15857.
- Samtsov AV, Bakulev AL, Khairutdinov VR, et al. Long-term data on the proposed adalimumab biosimilar BCD-057 in patients with moderate to severe psoriasis: a randomized controlled trial. PLoS One. 2022;17(2):e0263214. doi: 10.1371/journal.pone.0263214.
- Blauvelt A, Lacour JP, Fowler JF, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–631. doi: 10.1111/bjd.16890.