Abstract
Atopic dermatitis (AD), a chronic-relapsing inflammatory skin disorder, manifests with intense itching and eczematous lesions impairing quality of life. A heterogeneous population, and regional clinical practices for treating AD warrant the development of guidelines in Qatar. Therefore, guidelines for the management of moderate-to-severe AD in Qatar have been developed and discussed. Experts, including dermatologists and immunologists, used the Delphi technique for developing guidelines. Consensus was defined as ≥75% agreement or disagreement. AD is highly prevalent in primary and tertiary dermatology centers. AD-associated foot eczema and psoriasiform eczema are more frequent in Qatar than in Europe or USA. SCORing Atopic Dermatitis Index quantifies disease severity and itch. Dermatology Life Quality Index assesses the quality of life. Atopic Dermatitis Control Tool assesses long-term disease control. Moderate-severe AD benefits from new topicals like Janus-kinase-inhibitors or PDE4-inhibitors combined with phototherapy. Currently approved systemic agents are dupilumab, baricitinib, abrocitinib, and upadacitinib. New anti-IL-13 and anti-IL-31 therapies will soon be available. Patient education, allergy testing, and comorbidity consideration are critical in the management of AD. The expert panel established a comprehensive and pragmatic approach to managing moderate-to-severe AD, thereby assisting clinical decision-making for healthcare professionals in Qatar.
Introduction
Atopic dermatitis (AD) is an inflammatory dermatological disease characterized by intense itching and recurrent eczematous lesions (Citation1). It typically begins during infancy and gradually recurs or may persist to adolescence/adulthood with intermittent flare-ups and remissions (Citation2); however, a bimodal model with a second peak in middle-aged and older adults is not uncommon (Citation3). The disease can vary from mild-to-moderate to severe, necessitating different treatment regimens (Citation4).
The global prevalence rate of AD ranges from 2.7 to 20.1% in children and 2.1 to 4.9% in adults (Citation5,Citation6). Over the last 30 years, its prevalence surged 2- to 3-folds worldwide. In developed countries, the prevalence rate of AD is 1–3% in the older age groups, being slightly higher in elderly males (Citation7,Citation8). In the Middle East and North Africa (MENA) region, the prevalence of AD appears to have significantly increased, although exact epidemiological data is limited, and the design of the studies varies. The estimated prevalence rate of AD in MENA varies from 2.1% to 23.3% across countries and age groups (Citation9). A high prevalence of 30% has been found in a recent retrospective, cross-sectional study of 4521 patients from Qatar. The prevalence was found to be higher among boys (31.42%) than among girls (28.81%). The Qatari population demonstrated a greater prevalence of AD (34.4%) than the population that was non-Qatari. The prevalence was highest in children aged 6 months to one year (41.79%), followed by 8–12 years of age (32.8%), and was the least among 5–8 year olds (23.05%) (Citation10). Arid climatic conditions worsen AD due to heat, low humidity contributing to dryness of the skin (Citation9). In general, the epigenetic factors modulating AD in the population of MENA are poorly studied. Additionally, regional and ethnic diversity, as well as endotype specificities, may contribute to the varying prevalence, phenotypes, and therapy responses to AD globally, including MENA (Citation9,Citation11).
AD presents debilitating clinical symptoms and has several allergic comorbidities like allergic conjunctivitis, allergic rhinitis, food allergy, allergic asthma, nasal polyposis, along with bacterial, viral, and fungal dermatological infections (Citation12). The manifestations include diffuse erythematous patches, papulovesicular rash, xerosis (dryness), pruritus, and pain. Lichenification and prurigo are the main characteristics of chronic AD (Citation11,Citation12). All stages of AD affect patient’s quality of life (QoL) due to adverse symptoms, leading to deterioration in daily activities, and may lead to unemployment or suicide (Citation13,Citation14). Therefore, both QoL and the clinical manifestations of the patients should be taken into consideration while developing the control and management plan for AD.
European Academy of Dermatology and Venereology (EADV) emphasizes on severity-driven AD management according to SCORing Atopic Dermatitis (SCORAD) (Citation4). Topical glucocorticosteroids (TGCS) are the gold standard in the treatment of AD. When body areas more prone to atrophy are affected, the preferred treatment option includes the application of topical calcineurin inhibitors, such as pimecrolimus, tacrolimus, or crisaborole, for example. New topical JAK-inhibitors (e.g., delgocitinib, ruxolitinib) are effective for mild-to-moderate AD and will be a valuable addition to the armamentarium of mild-to-moderate AD therapy (Citation15). In patients with poorly controlled or moderate-to-severe AD, systemic agents should be used along with the topical treatment. Here, the guidelines from the American Academy of Dermatology Joint Task Force as well as the EADV Task Force suggest phototherapy as a systemic treatment strategy for AD. Immunosuppressants can also be selected to treat inadequately controlled or moderate-to-severe AD patients, along with topical agents and phototherapy (Citation16), the latter should not be combined with cyclosporine A (CSA). For holistic management, however, regional aspects for assessing, diagnosing, and treating AD are obligatory (Citation9) which may necessitate modifying international guidelines accordingly. For example, some topicals or phototherapy are not available in all countries, new systemic treatments may be ranked in a different way, and admissions may not be such an option as in other areas.
Several challenges are associated with the diagnosis, assessment, and management of AD in Qatar, including inconsistent application of diagnostic criteria to epidemiological analysis, paucity of universally accepted measures for the evaluation of disease severity and control, and unavailability of representative samples, which would enable the application of the guidelines to a larger population (Citation17). In addition, color-of-skin dependent complications including stigmatization are a troubling factor necessitating fast and sustainable management options.
The unmet clinical challenges in the diagnostic and therapeutic approaches for patients with AD in Qatar need to be reviewed for establishing an expert opinion. This can act as a reliable and up-to-date frame of reference for regional healthcare professionals to improve the diagnosis and management of AD. The present project was planned to evaluate the current regionally used methods for the assessment and local management options for moderate-to-severe AD in children, adolescents, and adults in Qatar.
Methods
Study design
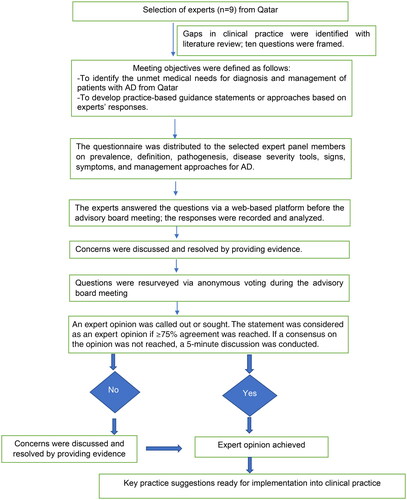
An advisory board meeting was held on 17 September 2021. The modified Delphi technique was used to conduct the survey and obtain expert opinions. The objectives of the study are as follows: (1) to identify unmet medical needs in the diagnosis and management of AD from the perspective of treating the patients of Qatar, (2) to obtain expert insights followed by voting on ten clinical questions pertaining to the definition, prevalence, pathogenesis, severity assessment tools, and treatment approaches for AD, and (3) to develop practice-based guidance statements or approaches based on experts’ responses and voting analysis.
The modified Delphi method (Citation18) has been used. The participants were asked to complete a questionnaire. The responses were used to generate a new set of questions, and the process was repeated until the majority of respondents arrived at the same answer. The methodology of a Delphi study includes three crucial aspects: (1) the participants are not aware of the identity of the other responders, which ensures an independent response, (2) the participants respond individually as some people may dominate the discussion, and (3) a mathematical voting process is used to permit the ranking of items.
Participants and procedure
The expert panel comprised nine renowned dermatologists and allergologists/immunologists from Qatar. The session occurred on a web-based platform, facilitating live interaction, topic organization, and voting on the topic discussed. Two scientific coordinators supervised the development of the study, including the enrollment of experts and administrative aspects of the advisory board meeting. They crafted pre-meeting questionnaires using peer-reviewed articles, systematic reviews, PubMed, and clinical insights. The process of development of the expert opinion is presented as an illustration in . The questionnaires were distributed among the selected expert panel members (). The statements by the group of dermatologists and immunologists in the meeting were used to develop an expert opinion on the management of moderate-to-severe AD.
Table 1. Overview of the responses of the expert panel to the survey questionnaire.
An ad hoc meeting was conducted on 8 August 2022 with the objective of the inclusion of recent updates in the treatment strategies for AD. Therefore, in this meeting, new treatment strategies for AD management, namely baricitinib, nemolizumab, abrocitinib, tralokinumab, and upadacitinib, were discussed.
Data analysis
Ten questions were considered for expert opinion, and a ≥ 75% agreement or disagreement among the experts was set a priori. Descriptive statistics were used to calculate the percentage of expert responses.
Results
The responses given by the expert panel to the survey questionnaire are presented in .
Expert opinion 1: Approval of European guidelines for defining AD
The experts approved the European guidelines for defining AD, which state that ‘AD is a chronic, inflammatory, and relapsing pruritic skin disease that generally occurs in families with atopic diseases like food allergy, bronchial asthma, or allergic rhino-conjunctivitis (Citation4).’ The experts also agreed that AD can be clinically diagnosed when three of the four major criteria and three of the twenty-three minor criteria of the Hanifin and Rajka Diagnostic Criteria (Citation19) are met.
Expert opinion 2: The prevalence rate of AD in adults ranges from 1 to 5%. Similarly, the prevalence of AD in adolescents and children ranges from 5 to 10 and >10%, respectively
The experts highlighted AD as a highly prevalent chronic inflammatory skin disorder. The prevalence rate of AD in adults, adolescents, and children as 1–5, 5–10, and >10%, respectively (Citation20–22). Globally, children (6–7 years) have 14.2% prevalence, while 13–14-year-old adolescents have 12.8% (Citation9). Over 20% of children in some countries have AD, with global variation (22). Around 70%–85% of adults showed childhood AD symptoms (Citation23). The experts cited the Barbarot et al. study, which reported adult AD prevalence in USA (4.9%), Canada (3.5%), Europe (4.4%), and Japan (2.1%). A regional variance was observed among European countries (2% in Germany and 8.1% in Italy) (Citation6). An ongoing study with a sample size of 36,494 patients with AD in Qatar, suggested the highest prevalence in 1–10 year olds (48.5%), marginally higher in males (50.3%) and more in non-Qatar patients (67%).
Expert opinion 3: Pruritus should be considered an essential feature in AD diagnosis
Experts concurred on the vital recognition of AD’s distinct features and clinical mimickers for accurate diagnosis and treatment initiation. Pruritus was considered as the main characteristic feature in the diagnostic criteria of AD. Recent research infers that inflammatory mediators (e.g., cytokines) activate sensory nerve endings, resulting in burning and itching sensations (Citation14,Citation24). Nocturnal itching leads to disturbed sleep and impaired QoL (Citation13,Citation14).
Expert opinion 4: The key cytokines IL-4, IL-13, and IL-31, and some other cytokines related to innate and adaptive immunity, and genetic modification in filaggrin play a predominant role in AD pathogenesis
Experts strongly agreed that AD is a multifactorial, genetic disease occurring when specific genes interact with environmental factors (Citation12). Allergens, may breach the skin barrier, bind to Langerhans cells, activate Th2 cells, and release IL-4 and IL-13, prompting IgE production and lowering antimicrobial peptides, which promotes microbial infections. T-helper 2 cells also release interleukin (IL)-31, responsible for causing pruritus in patients with AD (Citation12,Citation25).
Interleukin-31 alters the thickness of the epidermis and disrupts the skin barrier, increasing transepidermal water loss (Citation24). Thus, irritants and allergens may penetrate the epidermis, perpetuate inflammation, and cause infections. The intracellular signaling pathway, including the activator of transcription (STAT) and Janus kinase (JAK)-signal transducer, plays a crucial part in the upregulation of IL-4-, IL-13-, IL-31-, and IL-22-induced pro-inflammatory cytokines (Citation25). As a response to allergens penetrating the weakened skin barrier, the Langerhans cells activate the Th2 cells to release IL-4 and IL-13, especially during the acute phase, together with IL-31. In later phases, other cytokines, such as IL-22 (by Th22 cells) and IFNg come also into play. It has been suggested that the characteristic neurogenic itch of AD is directly promoted by IL-4 and IL-13 and via IL-31 by an indirect interaction (Citation26). The Th2-mediated inflammation releases IL-4, IL-13, and IL-31 that interrupt the barrier of the skin (Citation27). In addition, trigger factors stimulate the discharge of ‘alarmins’ (e.g., IL-25, IL-33, and TSLP) from the keratinocytes, which subsequently activate ILC2 cells and TH2 cells. ILC2 cells also release IL-31 which is involved in inflammation, skin barrier disruption, and pruritus (Citation24,Citation28). There is compelling evidence that the main mediators of pruritus in AD are IL-13, IL-4, and IL-31 (Citation1,Citation29,Citation30), which all can directly activate sensory nerves in the skin (Citation31). Recent studies have reported IL-17 and IL-22 expression in AD (Citation27,Citation32).
Expert opinion 5: The SCORAD test is recommended in routine clinical practice to assess the severity of AD
The experts identified SCORAD, developed by the European Task Force, as a tool for physicians to clinically assess AD severity in clinical practice. The experts also suggested that as both subjective and objective symptoms contribute to the severity of clinical manifestations, they should be evaluated for disease activity. As per the European guidelines, the expert group agreed that the disease could be classified as mild, moderate, and severe when SCORAD is <25, 25–50, and >50, respectively (Citation4).
Several studies have validated the reliability of the Eczema Area and Severity Index (EASI) as a well-validated tool for assessing the extent and AD severity. The four characteristics: (a) erythema, (b) infiltration/papulation, (c) excoriations, and (d) lichenification on four body areas, namely head, arms, body, and trunk, are assessed on a scale of 0–3, being absent to severe.
The Investigator Global Assessment (IGA) scale is a 5-point scale for the assessment of AD severity from 0 (clear) to 4 (severe). However, studies ascertaining its validity in daily practice are sparse; however, they have shown to be well suited for use in clinical trials (Citation33).
IGA is a rapid, well-assessed, and validated assessment tool in practice and clinical trials. Severity spans from global assessment from 0 to 4 (Citation33).
Expert opinion 6: The Dermatology Life Quality Index test is recommended in routine clinical practice to assess the severity of AD
There was a strong agreement on the measurement of QoL as it is an essential aspect of monitoring AD and the intervention efficacy in dermatology. The experts recommended the Dermatology Life Quality Index (DLQI) as the principal instrument to quantify the effect of AD on the QoL of patients, which was suggested by the Harmonizing Outcome Measures for Eczema initiative. Dermatology Life Quality Index was developed in 1994 as a survey specific to dermatology. The questionnaires have been translated into 11 languages and used in 80 countries. It is quick and easy to perform and score in routine clinical practice. It is widely recognized due to its psychometric properties and utility in clinical research. Dermatology Life Quality Index-validated score banding permits significant analysis of the responses. For example, score band 0–1 specifies that AD has no effect on a patient’s life, and 11–20 specifies that AD greatly affects the patient. This banding can assist in making informed clinical decisions (Citation34).
Expert opinion 7: The Atopic Dermatitis Control Tool (ADCT) is recommended in routine clinical practice to assess long-term disease control in patients with AD
The experts approved ADCT as a valid and reliable tool to measure patient-reported outcomes and assess patient-perceived control of AD. They also agreed that ADCT is a simplified and concise tool for the assessment of six symptoms of AD in the patient over the previous week. The six parameters consist of the severity of symptoms, duration of intense periods of itching, disturbed sleep, impaired daily activities, intensity of bother, and mood or troubled emotions. The score of the ADCT parameter ranges from 0 (minor symptoms) to 4 (severe symptoms), which demonstrates AD severity. The range of the total score is from 0 to 24, which is the aggregate of all the parameters (Supplementary Table 1) (Citation35).
Expert opinion 8: The case of AD has to be classified into mild/moderate/severe type in the first visit, and in the follow-up visit, the same case has to be classified into controlled or uncontrolled type
Based on their clinical experience, the experts agreed that a case of AD must be classified into mild/moderate/severe on the first visit.
Follow-ups for the patients were scheduled at 1, 2, 4, 8, 12, and 16 weeks (then depending on severity: Severe–once in 2 weeks; moderate–once in 4 weeks; mild–once in 6 weeks; no lesion–every 8 weeks), based on evidence from clinical trials, suggesting better treatment evaluation and promoting treatment adherence among patients (Citation36).
Patients with AD are classified as controlled and uncontrolled only in the subsequent visits based on the presence or absence of pruritus and clinical picture.
In many publications, controlled AD was classically defined as improving or stable, while uncontrolled AD could be expressed as changeable/deteriorating slowly/deteriorating rapidly; however, this assessment is too subjective and risks too much bias (Citation37). Therefore, the phone-app-calculator-accessible SCORAD is a well-validated and reliable tool for therapeutic success assessment. The calculator helps to assess patient treatment within 2 min.
The experts recommended that the following definition of controlled vs. uncontrolled patient based on ADCT score: ≥7 (not controlled) vs. <7 (controlled) be followed in Qatar. In patients with low EASI or SCORAD, but high itch scores, it is helpful to assess in addition the NRS11 itch score or Visual Analog Scale score, as well as DLQI. This ADCT classification is validated as a tool to assess controlled therapies and is chosen for practical reasons. However, because it is only validated thus far for treatment with dupilumab, a more general assessment tool is necessary (Citation38).
Expert opinion 9
In patients with inadequately controlled AD, the preferred systemic agents for use, either alone or with topical treatments, include the Food and Drug Administration (FDA)- or European Medicines Agency (EMA)-approved biologic dupilumab, a strongly recommended drug and/or with phototherapy as the preferred therapy. Alternative biologics that have received FDA and EMA approval include tralokinumab (another biologic not available in Qatar at the time this expert opinion was generated) and JAK inhibitors, including baricitinib, upadacitinib, and abrocitinib (baricitinib was the only available JAK inhibitor registered in Qatar for rheumatoid arthritis, whereas abrocitinib was not available for use in the country at the time this expert opinion was generated). For patients on JAK inhibitors, the screening and laboratory data are provided in (Citation39). The experts opined that monitoring JAK inhibitor and CSA antibodies can be done by obtaining the history of conjunctivitis, foreign body sensation, dry eye, eye itching or pain, and examination of the eye for redness. Conjunctivitis may be prevented by prescribing lubricating eyedrops from the start of therapy.
Table 2. Screening and laboratory monitoring for patients on JAK inhibitors and cyclosporine A.*
Additionally, other systemic immunosuppressants, such as azathioprine, mycophenolate mofetil, and methotrexate, are generally not FDA- or EMA-approved for the treatment of AD, except for CSA, which is indicated for treating severe psoriasis and nephrotic syndrome (off-label indication: CSA is licensed for short-duration treatment of adults with severe AD not responding to conventional therapy or in whom conventional therapy is inappropriate). Phototherapy may be considered for moderate-to-severe AD, either alone or combined with topical agents, except for CSA.
There was a strong agreement on the rank 1 sequence given to dupilumab (8/9; 88.88%), CSA (3/9 experts), azathioprine (1/9), phototherapy (2/9), and methotrexate (1/9) and a rank 2 sequence given to mycophenolate mofetil, tralokinumab (rank 2 sequence was given in the ad hoc meeting), and baricitinib. Experts noted limited clinical experience with baricitinib due to the recent introduction in Qatar and unavailability of tralokinumab. Therefore, most experts agreed that clinical experience in the prescription of baricitinib and tralokinumab is minimal. They proposed possible ranking revision based on emerging literature, comparing efficacy, safety, contraindications over time. Drugs used in systemic therapy for the treatment of moderate-to-severe AD in children and adolescents and the associated clinical evidence are given in (Citation40–52).
Table 3. Systemic therapies in children and adolescents with moderate-to-severe AD.
Details of drugs approved by the FDA and EMA in Qatar, their availability, and their recommended strength are listed in (Citation40–60).
Table 4. Approval status, availability, and strength of the recommendation of drugs used for the treatment of AD.
The summary of evidence on available therapies for AD is presented in Supplementary Table 2.
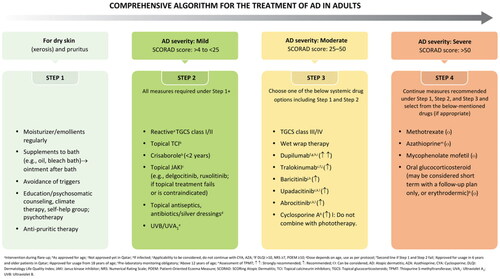
The comprehensive algorithm for the management of AD in adults is summarized in .
The strength of recommendation, wording, symbol, and interpretation are summarized in .
Table 5. Strength of recommendation, wording, symbol, and interpretation.
Expert opinion 10: Therapeutic patient education plays a vital role in the diagnosis, management, and control of AD
The experts postulated that therapeutic patient education (TPE) positively affects the course of the disease, autonomy, and QoL of patients and prevents complications associated with AD. Survey experts agreed that TPE could improve QoL and patient satisfaction. The experts also suggested educational programs (i.e., training programs and ‘eczema schools’) and video-based interventions, besides conventional therapy, for AD management. Moreover, it was agreed that eczema workshops and nurse-led programs are also useful.
Discussion
The management of AD requires long-term approaches consequent to the remitting, relapsing, and inflammatory characteristics of the disease (Citation1,Citation4). Atopic dermatitis has a greater prevalence among children than in adults (Citation2,Citation6). Underdiagnosis and lack of region-specific guidelines for AD are significant barriers to the effective management of the disease in Qatar. A committee meeting led by experts was conducted to identify the current challenges in diagnosing and managing AD in Qatar and provide optimal care to patients. Conclusions from the discussion were analyzed to formulate an expert opinion and develop new guidelines that would focus on implementing best practices and state-of-the-art diagnostic and therapeutic approaches in Qatar. Additionally, the published literature was reviewed, and the information gathered therein helped augment the formulation of the expert opinion. Later, an ad hoc meeting was conducted to update the recent treatment strategies for AD.
Experts agreed on the definition of AD as per European guidelines, the application of an objective tool (SCORAD index), and a subjective tool (DLQI) for quantification of the disease severity. Dupilumab was agreed to be the most recommended drug for moderate-to-severe AD when topical treatment failed. Other drugs of choice are baricitinib (currently approved for adults only), upadacitinib, abrocitinib, or tralokinumab. In Qatar, the only locally approved drug for managing AD is upadacitinib. Another systemic therapy or combined treatment with an agent, such as mycophenolate mofetil, CSA, azathioprine, or methotrexate was recommended in case of no improvement. Despite the lack of approval, evidence supports their clinical efficacy in the treatment of AD. In Qatar, patients are suggested to be referred to a specialized center when first- and second-line systemic agents are unavailable.
In this study, guidance from experts’ findings was brought together with the available literature to formulate an expert opinion for the diagnosis of AD and evaluate the efficacy of drugs, thereby facilitating better management of AD in Qatar. These experts’ opinions should aid other dermatologists and immunologists in managing AD and improving patients’ QoL.
The Pharmacovigilance Review Assessment Committee (PRAC) outlined measures to mitigate JAK inhibitor risks, including cardiovascular, thrombotic events, infections, and oncogenicity. JAK inhibitors should be prescribed if alternatives are exhausted, with caution, especially in patients at risk of blood clotting (Citation61). As limitations of this study include the unavailability of baseline knowledge regarding the management of AD in Qatar therefore educational campaigns on AD are warranted. This significant impediment needs to be addressed among clinicians in Qatar and the dissemination of established guidelines should be emphasized. Knowledge of the diagnosis and treatment of AD can assist clinicians in effective patient management and adherence. A post-release survey is needed to assess expert opinion impact on Qatar’s AD management. It is also required to recognize individual patient outcomes.
These guidelines are expected to assist physicians in clinical decision-making while enhancing patients’ comprehension and compliance. Consequently, significant clinical outcomes may be achieved.
Conclusion
The management of AD in Qatar is still challenging due to the lack of in-depth epidemiological data, sufficient assessment documentation for mild to moderate to severe cases at all age groups, analysis of atopic and non-atopic comorbidities; phenotypes and endotypes, for example. In Qatar, around 80% of adults with severe AD and pruritus (NRS11 10/10) appear to be controlled with dupilumab. Furthermore, the feasibility of treatments needs to be tailored to the health system with the availability of systemic therapy and phototherapy in private practices or primary centers, for example. Based on the prevalence, the treatment of the majority of cases in a tertiary center is not feasible. Consequently, it is necessary to bring together all physicians who treat AD and its comorbidities (e.g., allergology) to the table, and to optimize and streamline treatment options, specifically regional recommendations for the diagnosis and management of the disease, in Qatar. The present expert opinion establishes comprehensive and pragmatic approaches for effectively managing and treating AD in children, adolescents, and adult patients. New topical and systemic therapies are indispensable to treat all patients and their comorbidities holistically. For example, new therapies are only available in tertiary academic dermatology centers. Assessment scoring should be coordinated in private, primary, and tertiary centers.
The expert panel trusts that effectual diagnostic and therapeutic strategies play an important part in the management of patients. The present study highlights the importance of unmet needs for the adequate treatment of AD; emphasizes the recommendation of effective, safe, and approved systemic therapies for patients with AD; and encourages the need for larger national studies to understand the severity and impact of inadequate AD control. The categorization of patients into controlled or uncontrolled AD with pruritus as the index symptom could be a simplified and practical method in the assessment of prognosis in AD.
Author contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Authorship
All the listed authors are final and there are no changes to the authorship. We have included the rest of the contributors who do not qualify for authorship in the acknowledgments. Author contribution is also listed in the manuscript.
| Abbreviations | ||
| AD: | = | Atopic dermatitis |
| ADCT: | = | Atopic Dermatitis Control Tool |
| CSA: | = | cyclosporine A |
| DLQI: | = | Dermatology Life Quality Index |
| EADV: | = | European Academy of Dermatology and Venereology |
| EASI: | = | Eczema Area and Severity Index |
| FDA: | = | Food and Drug Administration or EMA - European Medicines Agency |
| IGA: | = | Investigator Global Assessment |
| JAK-inhibitors: | = | Janus-kinase |
| MENA: | = | Middle East and North Africa |
| PRAC: | = | Pharmacovigilance Review Assessment Committee |
| QoL: | = | quality of life |
| TGCS: | = | Topical glucocorticosteroids |
| TPE: | = | therapeutic patient education |
Supplemental Material
Download Zip (733.7 KB)Acknowledgments
Open access funding is provided by the Qatar National Library. Editorial support for this manuscript preparation was provided by BioQuest Solutions.
Disclosure statement
Martin Steinhoff acknowledged receipt of consultancy fees from AbbVie, Pfizer, Eli Lilly, Novartis, Sanofi, Biologix, Algorithm, Bayer, BMS, Chugai Pharmaceuticals, Toray Industries, Maruho, GSK, Kiniksa Pharmaceuticals, Leo Pharma, Pierre-Fabre, and Almirall. He received advisory board fees from AbbVie, Pfizer, Eli Lilly, Novartis, Sanofi, BMS, Chugai Pharmaceuticals, Toray Industries, Maruho, Leo Pharma, Pierre-Fabre, and Almirall. He also received research funding from AbbVie, Pfizer, Novartis, Bayer, BMS, Chugai Pharmaceuticals, Toray Industries, Maruho, Leo Pharma, Pierre-Fabre, and Almirall. Adel Mohamed Kamal, Ahmad Hazem, Hassan Riad, Mehdi Adeli, Mohamed Allam, Ra’ed Alsmadi, Waad Ibrahim, and Maryam Ali Al-Nesf do not have any conflicts of interest with respect to this manuscript.
Data availability statement
No underlying data were collected or produced for this manuscript.
Additional information
Funding
References
- Steinhoff M, Ahmad F, Pandey A, et al. Neuroimmune communication regulating pruritus in atopic dermatitis. J Allergy Clin Immunol. 2022;149(6):1–11. doi: 10.1016/j.jaci.2022.03.010.
- Abuabara K, Margolis DJ, Langan SM. The long-term course of atopic dermatitis. Dermatol Clin. 2017;35(3):291–297. doi: 10.1016/j.det.2017.02.003.
- Fishbein AB, Silverberg JI, Wilson EJ, et al. Update on atopic dermatitis: diagnosis, severity assessment, and treatment selection. J Allergy Clin Immunol Pract. 2020;8(1):91–101. doi: 10.1016/j.jaip.2019.06.044.
- Wollenberg A, Christen-Zäch S, Taieb A, et al. ETFAD/EADV Eczema Task Force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34(12):2717–2744. doi: 10.1111/jdv.16892.
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–428. doi: 10.1016/j.anai.2020.12.020.
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293. doi: 10.1111/all.13401.
- Patruno C, Napolitano M, Argenziano G, et al. Dupilumab therapy of atopic dermatitis of the elderly: a multicentre, real-life study. J Eur Acad Dermatol Venereol. 2021;35(4):958–964. doi: 10.1111/jdv.17094.
- Chello C, Carnicelli G, Sernicola A, et al. Atopic dermatitis in the elderly Caucasian population: diagnostic clinical criteria and review of the literature. Int J Dermatol. 2020;59(6):716–721. doi: 10.1111/ijd.14891.
- Al-Afif KAM, Buraik MA, Buddenkotte J, et al. Understanding the burden of atopic dermatitis in Africa and the Middle east. Dermatol Ther. 2019;9(2):223–241. doi: 10.1007/s13555-019-0285-2.
- Oommen BJ, Kadori WIM, Abdulmajeed J, et al. The prevalence of atopic dermatitis among children aged between 6 months and 12 years attending primary health care clinics in Qatar 2018–2019. Int J Res Med Sci. 2022;10(9):1872–1877. doi: 10.18203/2320-6012.ijrms20222261.
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2021;20:1–20.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi: 10.1038/s41572-018-0001-z.
- Simpson EL, Bieber T, Eckert L, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol. 2016;74(3):491–498. doi: 10.1016/j.jaad.2015.10.043.
- Steinhoff M, Schmelz M, Szabo IL, et al. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018;17(8):709–720. doi: 10.1016/S1474-4422(18)30217-5.
- de Graaf M, Janmohamed SR, Schuttelaar MLA, et al. Systemic treatment of children and adolescents with atopic dermatitis aged ≥2 years: a Delphi consensus project mapping expert opinion in northern Europe. J Eur Acad Dermatol Venereol. 2022;36(11):2153–2165. doi: 10.1111/jdv.18410.
- Eichenfield LF, Ahluwalia J, Waldman A, et al. Current guidelines for the evaluation and management of atopic dermatitis: a comparison of the joint task force practice parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol. 2017;139: s 49–S57. doi: 10.1016/j.jaci.2017.01.009.
- Lopez Carrera YI, Al Hammadi A, Huang YH, et al. Epidemiology, diagnosis, and treatment of atopic dermatitis in the developing countries of Asia, Africa, Latin America, and the Middle east: a review. Dermatol Ther. 2019;9(4):685–705. doi: 10.1007/s13555-019-00332-3.
- Linstone HA, Turoff M. 2002. The Delphi method: techniques and applications [Internet]. Available from: http://is.njit.edu/pubs/delphibook/delphibook.pdf
- Alakeel A, Al Sheikh A, Alraddadi AA, et al. Management of atopic dermatitis in adults in Saudi Arabia: consensus recommendations from the dermatological expert group. Clin Cosmet Investig Dermatol. 2022;15:1435–1445. doi: 10.2147/CCID.S357178.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8):S115–S123.
- Celakovska J, Josef B, Ettler K, et al. Atopic dermatitis in adolescents and adults: the evaluation of association with other allergic diseases and parameters. Food Agri Immunol. 2017;28(6):933–948. doi: 10.1080/09540105.2017.1320358.
- Bylund S, Kobyletzki LB, Svalstedt M, et al. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160. doi: 10.2340/00015555-3510.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(1):8–16. doi: 10.1159/000370220.
- Datsi A, Steinhoff M, Ahmad F, et al. Interleukin-31: the “itchy” cytokine in inflammation and therapy. Allergy. 2021;76(10):2982–2997. doi: 10.1111/all.14791.
- Yu S, Li Y, Zhou Y, et al. Immune mediators and therapies for pruritus in atopic dermatitis and psoriasis. J Cutan Immunol Allergy. 2019;2(1):4–14. doi: 10.1002/cia2.12049.
- Pappa G, Sgouros D, Theodoropoulos K, et al. The IL-4/-13 axis and its blocking in the treatment of atopic dermatitis. J Clin Med. 2022;11(19):5633. doi: 10.3390/jcm11195633.
- Sugaya M. The role of Th17-related cytokines in atopic dermatitis. Int J Mol Sci. 2020;21(4):1314. doi: 10.3390/ijms21041314.
- Alkon N, Bauer WM, Krausgruber T, et al. Single-cell analysis reveals innate lymphoid cell lineage infidelity in atopic dermatitis. J Allergy Clin Immunol. 2022;149(2):624–639. doi: 10.1016/j.jaci.2021.07.025.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–460. doi: 10.1016/j.jaci.2013.10.048.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171(1):217–228. doi: 10.1016/j.cell.2017.08.006.
- Steinhoff M, Oaklander AL, Szabó IL, et al. Neuropathic itch. Pain. 2019;160 Suppl 1(1):S11–S16. doi: 10.1097/j.pain.0000000000001551.
- Czarnowicki T, He H, Canter T, et al. Evolution of pathologic T-cell subsets in patients with atopic dermatitis from infancy to adulthood. J Allergy Clin Immunol. 2020;145(1):215–228. doi: 10.1016/j.jaci.2019.09.031.
- Ali F, Vyas J, Andrew F. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161. doi: 10.2340/00015555-3511.
- Simpson E, Eckert L, Gadkari A, et al. Validation of the atopic dermatitis control tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatol. 2019;19(1):15. doi: 10.1186/s12895-019-0095-3.
- Clinical Review Report: Dupilumab (Dupixent): (Sanofi-Aventis Canada Inc.): Indication: Moderate-to-severe atopic dermatitis (AD) [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2018. Appendix 5, Validity of Outcomes Measures. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539234/
- Patel NU, D’Ambra V, Feldman SR. Increasing adherence with topical agents for atopic dermatitis. Am J Clin Dermatol. 2017;18(3):323–332. doi: 10.1007/s40257-017-0261-5.
- Anderson P, Austin J, Lofland JH, et al. Inadequate disease control, treatment dissatisfaction, and quality-of-life impairments among us patients receiving topical therapy for atopic dermatitis. Dermatol Ther. 2021;11(5):1571–1585. doi: 10.1007/s13555-021-00580-2.
- Pariser DM, Simpson EL, Gadkari A, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the atopic dermatitis control tool (ADCT©). Curr Med Res Opin. 2020;36(3):367–376. doi: 10.1080/03007995.2019.1699516.
- Samuel C, Cornman H, Kambala A, et al. A review on the safety of using jak inhibitors in dermatology: clinical and laboratory monitoring. Dermatol Ther. 2023;13(3):729–749. doi: 10.1007/s13555-023-00892-5.
- Product prescribing information of dupilumab [Internet]; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Summary of product characteristics of dupilumab [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf
- Beck LA, Deleuran M, Bissonnette R, et al. Dupilumab provides acceptable safety and sustained efficacy for up to 4 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2022;23(3):393–408. doi: 10.1007/s40257-022-00685-0.
- Product prescribing information of upadacitinib [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf
- Summary of product characteristics of upadacitinib [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/rinvoq-epar-product-information_en.pdf
- Katoh N, Ohya Y, Murota H, et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (rising up): an interim 24-week analysis. JAAD Int. 2022;6:27–36. doi: 10.1016/j.jdin.2021.11.001.
- Summary of product characteristics abrocitinib [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf
- Prescribing information of abrocitinib [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Barcitinib FDA labelling information [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207924s000lbl.pdf
- Summary of product characteristics of barcitinib [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898.
- Tralokinumab prescribing information [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
- Summary of product characteristics of tralokinumab [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/adtralza-epar-product-information_en.pdf
- Prescribing information of cyclosporin [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050715s027,050716s028lbl.pdf
- Summary of product characteristics of cyclosporin [Internet]. Available from: https://www.ema.europa.eu/en/documents/referral/sandimmun-neoral-article-30-referral-annex-iii_en.pdf
- Methotrexate tablet [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/008085s066lbl.pdf
- Summary of product characteristics of methotrexate [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/jylamvo-epar-product-information_en.pdf
- Product monograph of azathioprine [Internet]. Available from: https://pdf.hres.ca/dpd_pm/00059993.PDF
- Summary of product characteristics of azathioprine [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/jayempi-epar-product-information_en.pdf
- Mycophenolate mofetil capsules [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050722s021,050723s019,050758s019,050759s024lbl.pdf
- Summary of product characteristics of mycophenolate mofetil [Internet]. Available from: https://www.ema.europa.eu/en/documents/product-information/mycophenolate-mofetil-teva-epar-product-information_en.pdf
- Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 24–27 Oct 22 [Internet]. Available from: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-24-27-october-2022