Abstract
Purpose: Vitiligo is an idiopathic depigmenting skin disorder. The study compares the efficacy of topical tacrolimus 0.1% with calcipotriol/betamethasone dipropionate in vitiligo patients receiving NB-UVB treatment.
Materials and methods: Forty-one adult patients with generalized type vitiligo were recruited. Patients were assigned to phototherapy and then classified into either group one (20 patients), receiving calcipotriol/betamethasone dipropionate cream (D group), or group two (21 patients), receiving tacrolimus 0.1% ointment (T group). They were followed-up at 3 and 6 months.
Results: The D group witnessed an increase in the repigmentation area from 35.4% in the third month to 54.7% in the sixth month (p = 0.001) and the T group from 32.2% to 45.6% (p = 0.011). However, the differences between the treatment groups were not statistically significant. Body sites demonstrated different levels of improvement ranging from the highest in the face to the lowest in the Hand & Feet with the other body sites in between. A negative correlation was identified between the duration since diagnosis and the response to D treatment (3 months: r = −0.612, p = 0.007; 6 months: r = −0.755, p = 0.001).
Conclusions: Although both combinations are efficacious, they did not significantly differ in efficacy at three and six months follow-up points.
Clinical trial registration: The study was registered at clinicaltrials.gov (NCT04440371).
Introduction
Vitiligo is a dermatological disorder of uncertain etiology, characterized by the appearance of white macules due to the selective loss or dysfunction of melanocytes, making it the most prevalent skin depigmentation condition (Citation1,Citation2). Globally, it affects approximately 0.4%–2% of the population (Citation2). The condition is frequently linked with stigmatization and a significant decrease in quality of life (QoL), which is exacerbated in patients with more extensive or rapidly progressing disease later in life (Citation3). Moreover, vitiligo is associated with various psychological conditions, including depression, which appears to have a linear relationship with the condition’s duration, and stress, which is prevalent among patients and may exacerbate the disease (Citation4).
While autoimmunity is widely accepted as the primary mechanism underlying vitiligo development, several other factors have been proposed, including genetic predisposition, oxidative stress, production of inflammatory mediators, and melanocyte detachment processes (Citation1). The ‘convergence theory’ posits that multiple mechanisms may interact synergistically, contributing to the loss of melanocytes in vitiligo patients (Citation1). Vitiligo is classified into two main categories: segmental and non-segmental vitiligo. Non-segmental vitiligo can manifest bilaterally and encompasses focal, mucosal, acrofacial, generalized, universal, and rare variants, whereas segmental vitiligo typically presents unilaterally, affecting a single dermatome (Citation5).
To date, there is no fully effective treatment for vitiligo. However, various treatment modalities have been reported, including phototherapy, topical corticosteroids, topical calcineurin inhibitors, and surgical interventions (Citation6). Phototherapy, either as a standalone treatment or in combination with other topical or systemic medications, is among the most commonly utilized therapies. Generally, combination therapies provide greater benefits than monotherapies (Citation1,Citation6). For instance, the combination of narrowband ultraviolet B (NB-UVB) with topical medications, such as tacrolimus 0.1% or calcipotriol/betamethasone dipropionate, has demonstrated superior efficacy compared to monotherapy (Citation7,Citation8). A randomized controlled trial comparing the efficacy of NB-UVB combined with tacrolimus 0.1% to NB-UVB alone in vitiligo patients found that the combined approach was more effective (Citation7). Similarly, the combination of NB-UVB, calcipotriol, and betamethasone yielded better results than NB-UVB alone (Citation8). The primary objective of this study is to compare the efficacy of topical tacrolimus 0.1% with calcipotriol/betamethasone dipropionate in vitiligo patients receiving NB-UVB treatment.
Materials and methods
Participants and settings
Participants were patients visiting our dermatology outpatient clinics at King Abdullah University Hospital, Irbid, Jordan, between (June 2020) and (March 2023). We recruited Forty-one adult patients (age 18 or above) with generalized type vitiligo with a Body Surface Area (BSA) of 10% or more. The diagnosis was confirmed both clinically and using wood’s lamp examination. Moreover, patients on treatment for vitiligo were given a one-month wash-off before enrollment in the study. Pregnant or breast-feeding women and patients with autoimmune diseases or previously failed to respond to phototherapy were excluded. The participants were informed about the purpose of the study, guaranteed confidentiality, and the possibility of withdrawing from the study at any time.
Study design and procedure
This is a randomized clinical trial in which patients were randomly classified into two treatment groups. All participants in the trial had three weekly phototherapy sessions assigned to them by their doctors at the beginning. Patients were then randomly classified into either group one (20 patients), receiving calcipotriol/betamethasone dipropionate cream (D group) once daily, or group two (21 patients), receiving tacrolimus 0.1% ointment (T group) twice daily on top of phototherapy. Patients were then scheduled to follow-up visits at 3 and 6 months from the start of therapy to evaluate the efficacy of treatments (the percentage of repigmentation area). The level of improvement at six months compared to baseline was classified into five groups according to the percentage of repigmentation as follows: excellent (76%–100%), moderate (51%–75%), mild (26%–50%), minimal (1%–25%), or no response.
Statistical analysis
Descriptive statistics provide a summary of participants’ demographic and clinical characteristics. Means and t-tests were used to compare continuous data of the treatment groups. Chi-square, medians, and percentages were used for categorical data. All analyses were carried out using SPSS version 25.0 (IBM Corp, Armonk, NY, USA), and the significance level was 5%.
Ethical considerations
The study was approved by the Institutional Review Board (IRB) of Jordan University of Science and Technology (83/2017). All patients had informed consent before participating in the trial, and their confidentiality was assured.
Results
In this trial, 20 patients received calcipotriol/betamethasone cream, and 21 received tacrolimus 0.1% ointment alongside NB-UVB. The males comprised 45% of the calcipotriol/betamethasone group and 57% of the tacrolimus 0.1% group, and the range of age for the patients was from 18 to 80 years old, with a mean age of 30 and 35 years for both groups, respectively. The duration of the disease since diagnosis ranged from 1 month to 20 years, with a mean duration of 4 years. The mean percentage of affected Body Surface Area with vitiligo at baseline was 14% and 11% for the calcipotriol/betamethasone and tacrolimus 0.1% groups, respectively.
The baseline demographic and clinical attributes, including the average vitiligo-affected body surface area (BSA) percentage, showed no discernible disparity between the two groups. Over the course of three and six months of therapy, both groups exhibited a consistent augmentation in the mean repigmentation area percentage. Nonetheless, the distinction between the D and T groups was not statistically substantial at the individual time frames ().
Table 1. demographic and clinical characteristics of the participants.
Notably, each group exhibited substantial statistical improvements. The D group witnessed an increase in the repigmentation area from 35.4% in the third month to 54.7% in the sixth month (p = 0.001). The T group followed a similar trajectory, escalating from 32.2% to 45.6% (p = 0.011). However, these differences between the treatment groups were not statistically significant, with p values of 0.625 after three months and 0.251 after six months ().
Table 2. Efficacy of each treatment and the differences in the efficacy of both treatment groups at three and six months from baseline.
Upon categorizing responses based on repigmentation areas, 60% of subjects in the D group and 28% in the T group achieved moderate to excellent repigmentation following six months of treatment ().
Table 3. Repigmentation percentage in the treatment groups after 6 months from baseline.
The involved body sites were face, trunk, upper limb (UL), lower limb (LL), and hands and feet of 17, 25, 26, 30, 32 patients, respectively. The response of treatment improved significantly across all body sites for both treatments together between the third and sixth months with p values of 0.020, 0.001, 0.001, 0.004, and 0.014 for face, trunk, UL, LL, and hands and feet, respectively. Furthermore, there was no significant difference in the response between the treatment groups at either follow-up point. However, body sites demonstrated different levels of improvement ranging from the highest in the face (71.4%) for D group and (80%) for T group to the lowest in the hand and feet (13.3%) for D group and (5.9%) for T group at six months with the other body sites in between (). Subsequent analysis of the response to therapies based on the type of treatment for various body sites revealed significant improvement in the trunk (p = 0.002), upper limb (p = 0.004), and lower limb (p = 0.01) regions for the D group and the upper limb (p = 0.034) region for the T group within the span of three to six months of therapy ( and ).
Figure 1. The repigmentation in the trunk (breast) of a female patient after 3 months and 6 months of treatment with calcipotriol/betamethasone cream (combined with VBUVB).
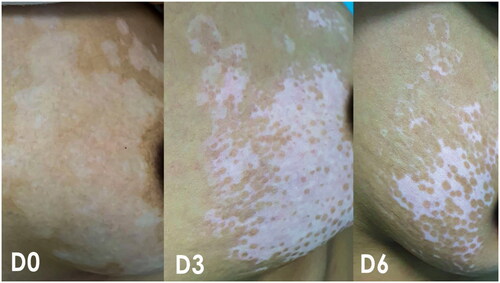
Figure 2. The repigmentation in the LL and foot of a male patient after 3 months and 6 months of treatment with tacrolimus 0.1% ointment (combined with VBUVB).
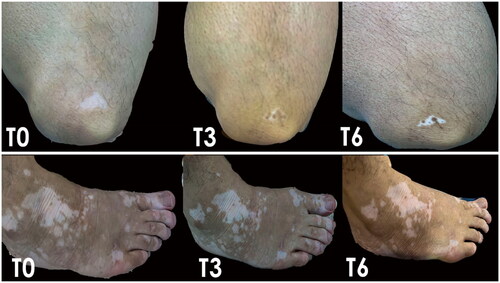
Table 4. Percentage of patients who showed excellent response to treatments in different body sites.
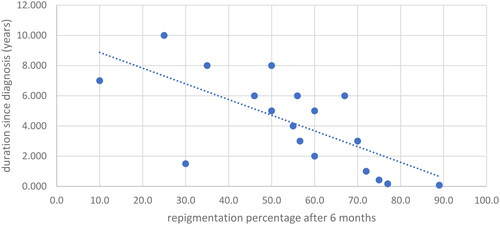
The study scrutinized several factors that could influence treatment outcomes. Calcipotriol/Betamethasone demonstrated significantly increased efficacy in patients younger than 33 years after three months of treatment (p = 0.038), though this variance was not sustained at six months. Intriguingly, a negative correlation was identified between the duration of the disease since diagnosis and the response to D treatment at both time points (3 months: r = −0.612, p = 0.007; 6 months: r = −0.755, p = 0.001) ( and )
Figure 3. Correlation of duration since diagnosis with repigmentation percentage after 3 months for the D group.
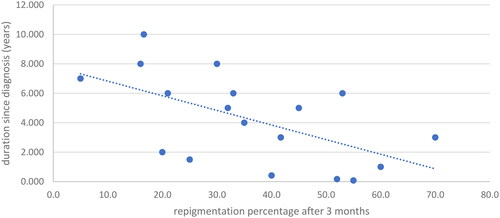
Figure 4. Correlation of duration since diagnosis with repigmentation percentage after 6 months for the D group.
Skin type appeared to play a role in treatment response, with type III skin demonstrating a diminished response to both therapies. However, these variances were not statistically significant. Finally, no remarkable effect on treatment response was observed concerning gender and Vitamin D or B12 levels in either treatment group at any time.
In essence, both calcipotriol/betamethasone cream and tacrolimus 0.1% ointment displayed their effectiveness in treating vitiligo, with notable enhancements observed within each group throughout the therapy. The research provides valuable insights into factors influencing the therapeutic response and may steer future clinical decision-making.
Discussion
As per our understanding, this represents the initial research effort to contrast the performance of tacrolimus 0.1% ointment and calcipotriol/betamethasone cream in vitiligo patients undergoing NB-UVB treatment. In our results, the calcipotriol/betamethasone group achieved numerically greater repigmentation percentages compared to the tacrolimus group, although this difference did not reach statistical significance. Similarly, more patients demonstrated excellent and moderate responses to calcipotriol/betamethasone than tacrolimus. In contrast to our study, a clinical trial compared the efficacy of topical tacrolimus to that of calcipotriol plus betamethasone, but in addition to microneedling instead of NB-UVB. It involved 25 patients, and two bilateral patches were identified on each side of their bodies. The left side received calcipotriol/betamethasone, while the right side received tacrolimus. The left side demonstrated a better response, with a 72% improvement compared to 54% on the right side after six months of treatment. Additionally, excellent improvement was achieved in 60% and 32% of the patients in the calcipotriol/betamethasone and tacrolimus groups, respectively (Citation9). Contrary to our study, these differences were statistically significant. Therefore, future studies are needed to compare the head-to-head efficacy of calcipotriol/betamethasone combined with NB-UVB versus microneedling. Our results are consistent with the previous research concerning the body site’s responses to treatment. The lesions typically show a better response in the face and neck followed by the trunk and extremities then, the least responding hands and feet. Additionally, younger patients and shorter onset of disease are associated with better results (Citation9,Citation10).
In our study, vitamin D was normal in six patients, while the rest had insufficient or low levels. vitamin D is involved in melanogenesis in human melanocytes (Citation11). According to a case-control study that compared vitamin D levels in vitiligo patients versus controls in Jordan, the low levels of vitamin D are similar among patients and controls taken from general populations. Its deficiency is not associated with vitiligo (Citation12). Our results showed that vitamin D level did not affect the response to treatment for either treatment group across the period of the study. In vitiligo treatment, multiple factors should be considered, such as patient’s age, disease duration, rate of spread, sites and extent of skin affected, availability of treatment options, and general health condition, among other relevant factors (Citation6). The effectiveness of NBUVB as a monotherapy is well-established in the literature. It proved to be a useful and well-tolerated therapy for vitiligo as its side effects are minimal. In a trial that involved 14 general vitiligo patients aged from 12 to 56 who received NBUVB alone three times per week, 71% of patients showed more than 75% improvement percentages after one year of treatment (Citation13). Multiple medications have been added to NBUVB as combination treatments and among them are tacrolimus and betamethasone which we compared in our study.
Tacrolimus as an effective treatment for vitiligo was first reported in 2002 with moderate to excellent repigmentation in children and adults (Citation14). It is an immunomodulator agent that inhibits the overactive T-cells and blocks transcription for the genes that encode many cytokines, especially IL-10, IFN-g, and TNF-a, which are involved in the pathogenesis of vitiligo (Citation15). Tacrolimus as a monotherapy for vitiligo is thoroughly reported in the literature. For instance, a multicenter randomized controlled trial that examined tacrolimus efficacy in treating facial vitiligo showed a repigmentation percent of 65% in tacrolimus-treated patients compared to 0% in the vehicle group after 24 weeks (Citation16).
Furthermore, another randomized controlled trial evaluated the efficacy of tacrolimus at 0.03% in 31 patients with non-segmental vitiligo and found a repigmentation percentage of 45% after 24 weeks (Citation17). Generally, combination therapy regimens are superior to monotherapies (Citation6,Citation18). According to a systematic review of the Cochrane system, tacrolimus once daily combined with NB-UVB is significantly better than NB-UVB alone, and the percentage of reduction in lesion area was 42.1% and 29%, respectively. Furthermore, the reduction in lesion area is correlated with the number of tacrolimus applications but not with the frequency of UV sessions (Citation6). These results are consistent with our study, which demonstrated a significant improvement in the repigmentation percentage from 32.2% after three months to 45.6% after six months from baseline. Besides, 19% and 10% of patients showed excellent and moderate responses respectively after six months.
Calcipotriol, a synthetic form of Vitamin D, is used in vitiligo therapy either as monotherapy or combined with other treatments like betamethasone. The efficacy obtained from combining calcipotriol with narrowband UVB or combining calcipotriol, betamethasone, and narrowband UVB is comparable, but the combinations have superior efficacy than NB-UVB alone (Citation8). Moreover, adding calcipotriol to the combination is beneficial in decreasing the side effects linked to betamethasone (Citation19). Similar results were obtained from our study, where the combination of calcipotriol/betamethasone and NB-UVB significantly improved repigmentation percentages from 35.4% after three months to 54.7% after six months of treatment. Besides, 15% and 45% of patients showed excellent and moderate responses after six months.
Our results imply that multiple clinical factors should be considered, including the side effects of each treatment. Corticosteroids such as betamethasone have side effects that range from skin atrophy, telangiectasia, and cutaneous infections to systemic effects (Citation20). On the other hand, tacrolimus induces minimal to mild side effects such as burning, pruritus, perioral folliculitis, and erythema. These side effects subsided spontaneously and did not lead to treatment discontinuation (Citation15,Citation17,Citation21).
Limitations
The patients were not blinded to the treatment which poses the risk of performance bias, and investigators were not blinded to the outcome indicating a risk of detection bias although, the same investigators measured the outcome both by patient examinations and photos. The study sample was heterogenous with a wide age range (from 18 to 80 years old), skin type (II, III, and IV), and body sites (face, trunk, upper limbs, lower limbs, hands, and feet). We analyzed data in subsets stratifying it by age, skin type, affected anatomical site, and type of treatment. This led to a smaller sample in each subgroup and may have influenced the subsequent analysis of data. Future research should recruit adequate samples of each category of vitiligo patients to enhance the statistical analysis.
Conclusion
The combinations of NB-UVB with tacrolimus 0.1% ointment or with calcipotriol/betamethasone dipropionate are efficacious in treating general vitiligo in adults. However, the treatments did not significantly differ in efficacy at three and six months follow-up points.
Author contributions
Conceptualization, D.S.,F.Q.,J.M., Methodology, D.S.,S.B.,A.F.,S.S., Formal Analysis S.B.,A.F.,S.S., Data collection D.S.,F.Q.,J.M.,A.T.,D.Sa., Resources D.S.,F.Q.,J.M.,A.T.,D.Sa., Writing original manuscript D.S.,F.Q.,J.M.,S.B.,A.F.,S.S.,A.T.,D.Sa., Review & Editing D.S.,F.Q.,J.M.,S.B.,A.F.,S.S.,A.T.,D.Sa., Supervision of study conduct D.S.,F.Q., Project administration and funding D.S., F.Q.
Disclosure statement
The authors declare no conflict of interest in relation to this study.
Data availability statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon request.
Additional information
Funding
References
- Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):1–6. doi: 10.1159/000506103.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x.
- Bibeau K, Pandya AG, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36(10):1831–1844. doi: 10.1111/jdv.18257.
- Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther. 2020;33(3):e13418.
- Ezzedine K, Lim H, Suzuki T, et al. Revised classification/nomenclature of vitiligo and related issues: the vitiligo global issues consensus conference. Pigment Cell Melanoma Res. 2013;25:1–28.
- Whitton M, Pinart M, Batchelor JM, et al. Evidence-based management of vitiligo: summary of a cochrane systematic review. Br J Dermatol. 2016;174(5):962–969. doi: 10.1111/bjd.14356.
- Nordal EJ, Guleng GE, Rönnevig JR. Treatment of vitiligo with narrowband-UVB (TL01) combined with tacrolimus ointment (0.1%) vs. placebo ointment, a randomized right/left double-blind comparative study. J Eur Acad Dermatol Venereol. 2011;25(12):1440–1443. doi: 10.1111/j.1468-3083.2011.04002.x.
- Akdeniz N, Yavuz IH, Gunes Bilgili S, et al. Comparison of efficacy of narrow band UVB therapies with UVB alone, in combination with calcipotriol, and with betamethasoneand calcipotriol in vitiligo. J Dermatolog Treat. 2014;25(3):196–199. doi: 10.3109/09546634.2013.777381.
- Ibrahim ZA, Hassan GF, Elgendy HY, et al. Evaluation of the efficacy of transdermal drug delivery of calcipotriol plus betamethasone versus tacrolimus in the treatment of vitiligo. J Cosmet Dermatol. 2019;18(2):581–588. doi: 10.1111/jocd.12704.
- Sisti A, Sisti G, Oranges CM. Effectiveness and safety of topical tacrolimus monotherapy for repigmentation in vitiligo: a comprehensive literature review. Ann Bras Dermatol. 2016;91(2):187–195. doi: 10.1590/abd1806-4841.20164012.
- AlGhamdi K, Kumar A, Moussa N. The role of vitamin D in melanogenesis with an emphasis on vitiligo. Indian J Dermatol Venereol Leprol. 2013;79(6):750–758. doi: 10.4103/0378-6323.120720.
- Alshiyab DM, Al-Qarqaz FA, Heis LH, et al. Assessment of serum vitamin D levels in patients with vitiligo in Jordan: a case-control study. Dermatol Res Pract. 2019;2019:2048409–2048404. doi: 10.1155/2019/2048409.
- Kanwar AJ, Dogra S, Parsad D, et al. Narrow-band UVB for the treatment of vitiligo: an emerging effective and well-tolerated therapy. Int J Dermatol. 2005;44(1):57–60. doi: 10.1111/j.1365-4632.2004.02329.x.
- Grimes PE, Soriano T, Dytoc MT. Topical tacrolimus for repigmentation of vitiligo. J Am Acad Dermatol. 2002;47(5):789–791. doi: 10.1067/mjd.2002.126250.
- Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51(1):52–61. doi: 10.1016/j.jaad.2003.12.031.
- Seneschal J, Duplaine A, Maillard H, et al. Efficacy and safety of tacrolimus 0.1% for the treatment of facial vitiligo: a multicenter randomized, double-blinded, vehicle-controlled study. J Invest Dermatol. 2021;141(7):1728–1734. doi: 10.1016/j.jid.2020.12.028.
- Saleh R, Ahmed AAE, M Abd-Elmagid W. Efficacy of topical tacrolimus 0.03% monotherapy in the treatment of non-segmental vitiligo: a randomized, controlled trial. J Cosmet Dermatol. 2021;20(12):3943–3952. doi: 10.1111/jocd.14041.
- Zhu B, Liu C, Zhang L, et al. Comparison of NB-UVB combination therapy regimens for vitiligo: a systematic review and network meta-analysis. J Cosmet Dermatol. 2023;22(3):1083–1098. doi: 10.1111/jocd.15534.
- Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J Eur Acad Dermatol Venereol. 2006;20(3):269–273. doi: 10.1111/j.1468-3083.2006.01420.x.
- Aliyev R, Finsterer J. Systemic toxicity to betamethasone ointment. Clin Case Rep. 2020;8(9):1635–1637. doi: 10.1002/ccr3.2957.
- Hartmann A, Bröcker EB, Hamm H. Occlusive treatment enhances efficacy of tacrolimus 0.1% ointment in adult patients with vitiligo: results of a placebo-controlled 12-month prospective study. Acta Derm Venereol. 2008;88(5):474–479. doi: 10.2340/00015555-0464.

