Abstract
Purpose: Although psoriasis and atopic dermatitis (AD) were for decades considered to be opposing diseases, it is now known that these skin conditions can coexist or even overlap in the same individual. Especially when using modern drugs with targeted IL inhibition, the balance between Th1 and Th2 immunity can be disturbed. In line with it, numerous clinical cases of AD have been induced by antipsoriatic biologics (e.g., TNF-alpha, IL-23, or IL-17 inhibitors), and IL-4-/IL-13 inhibition by dupilumab also resulted in paradoxical psoriasis in patients with AD.
Materials and methods: Herein, we describe a case of psoriasis vulgaris in a patient with intrinsic AD after systemic treatment with the anti-IL-13 antibody tralokinumab.
Results: We present a 36-years-old male patient with a severe course of an intrinsic atopic dermatitis and dyshidrotic hand eczema. He responded well to the therapy with tralokinumab. However, about 7 months after the start of anti-IL-13 treatment the patient developed psoriasiform lesions. The drug was then discontinued. Currently, the patient is receiving topical therapy with topical corticosteroids and calcineurin inhibitors with stable course of psoriasis and AD.
Conclusions: This case suggests, that not only a dual IL-4-/IL-13-blockade, but also a selective IL-13-inhibition is able to skew immune responses toward IL-17 cytokine pathway-related disease. However, no clinical scores exist to predict the development of paradoxical psoriasis in patients with AD during therapy with biologics.
Dear Editor,
For many decades psoriasis and atopic dermatitis (AD) have been considered as opposite conditions driven by Th1-/Th17- and Th2-mediated immunity, respectively [Citation1]. However, these diseases may coexist or even overlap in the same individual [Citation2]. For example, in Asian AD patients or children with intrinsic AD enhanced co-expression of IL-17 and interferon-γ was observed, and clinical and histopathological features were similar to those of psoriasis vulgaris [Citation1]. In line with this, numerous clinical cases of AD were induced by anti-psoriatic biologics (e.g. TNF-alpha-, IL-23- or IL-17-inhibitors) [Citation3]. Moreover, IL-4-/IL-13-inhibition by dupilumab can induce paradoxical psoriasis in patients with AD [Citation4]. We describe herein a case of psoriasis vulgaris in a patient with AD following systemic treatment with the anti-IL-13-antibody tralokinumab.
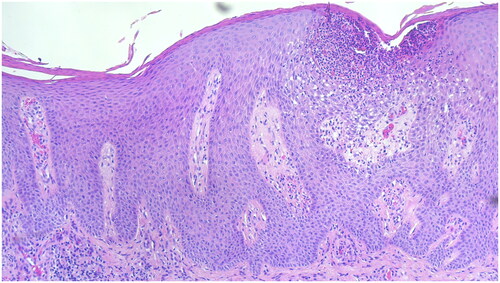
We report on a 36-year-old male who suffered from recurrent AD and dyshidrotic hand eczema since the age of ten. There were no known allergies or extrinsic trigger factors. His previous therapies included daily use of moisturizers, topical therapy with corticosteroids (TCS) and calcineurin inhibitors (TCI), that led to only inadequate improvement of the skin condition (). Therefore, subcutaneous therapy with dupilumab 300 mg every two weeks was introduced. After the initial improvement of both AD and dyshidrotic hand eczema the patient experienced a new exacerbation of the disease. There were no clinical patterns of psoriasis or psoriasiform eruption at this time. Therefore, five months after the initiation of dupilumab treatment his therapy was changed to oral baricitinib 4 mg per day. Due to the stable skin condition the baricitinib dose was reduced to 2 mg per day at about 6 months of treatment. Another 6 months later the patient experienced a new severe relapse of the AD, that couldn’t be controlled by increasing the baricitinib dose. Thus, the systemic therapy was changed to subcutaneous tralokinumab 300 mg every two weeks. After the initiation of tralokinumab treatment the patient experienced a stable remission of both AD and dyshidrotic hand eczema. Approximately seven months after start of tralokinumab the patient developed new skin lesions on the knees, elbows and scalp (). We diagnosed and histologically confirmed () a paradoxical psoriasis vulgaris and tralokinumab therapy was discontinued.
Figure 1. Clinical presentation of severe AD prior to the initiation of systemic therapy with erythematous, dry skin and scratch marks on the back (A) and neck (B). the SCORAD (SCORing atopic dermatitis) was 65.
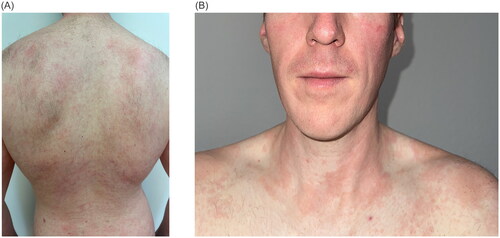
Figure 2. Clinical presentation of paradoxical tralokinumab-induced psoriasis with red, scaly plaques on the elbows (A) and erythematous patches at the hairline covered by silvery-white scales (B).
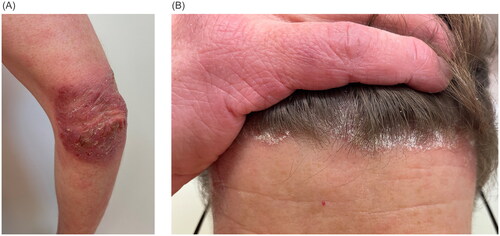
Figure 3. Histology of a skin biopsy from the lesion on the elbow with hyper- and parakeratosis, acanthosis, papillomatosis and subcorneal Munro’s microabscess, that is typical for psoriasis. Hematoxylin-eosin staining, ×100.
Different mechanisms have been suggested to play a role in the development of a paradoxical psoriasis during therapy with dupilumab. As IL-4 is a negative regulator of Th17 cell differentiation, its inhibition promotes a shift toward Th17 predominance and IL-17A und IL-17F production, which is characteristic for psoriatic inflammation [Citation5]. The inhibition of the IL-4 activity has been suggested to be crucial for the development of paradoxical dupilumab-induced psoriasis, as patients with this immune phenomenon may achieve remission of both AD and psoriasis upon therapy with tralokinumab [Citation6]. However, this mode of action doesn’t explain a new onset of psoriasis in our patient on the treatment with tralokinumab, an anti-IL-13-antibody, that has no effect on IL-4 activity. Interestingly, diminished IL-13 levels and de novo IL-17A expression were found using RNA in situ hybridization in a biopsy of a dupilumab-associated psoriasiform eruption [Citation7], thus this phenomenon might be explained by a Th2-Th1/Th17 immune imbalance. Some authors reported also about COVID-19 infection as a possible co-factor for development of psoriasis during anti-IL-4-/IL-13-treatment [Citation8]. Our patient, however, denied SARS-CoV-2 infection or COVID-19 vaccination in temporal relation to the onset of psoriatic lesions.
To the best of our knowledge, we describe a rare case of paradoxical tralokinumab-induced psoriasis in a patient with AD. This case suggests, that not only a dual IL-4-/IL-13-blockade, but also a selective IL-13-inhibition is able to skew immune responses toward IL-17 cytokine pathway-related disease. So far, no clinical scores exist to predict the development of paradoxical psoriasis in patients with AD or paradoxical eczema in patients with psoriasis vulgaris. However, the intrinsic phenotype of AD in our patient could represent a hint for the possible switching from AD to psoriasis. Further research is needed to gain more evidence on clinical features of patients at risk.
In about half of the cases of paradoxical psoriasis on dupilumab reported in the literature the culprit therapy was discontinued, which, however, doesn’t necessarily lead to the resolution of this skin condition [Citation4]. Although a combination of dupilumab and anti-psoriatic biologics has been reported to be safe and effective for the management of concomitant AD and psoriasis [Citation9], this approach doesn’t appear to the authors as optimal solution to restore the Th2-Th1 balance. Reasonable choices for overlapping psoriasis and AD could represent TCS and TCI, narrow band UVB phototherapy, conventional oral medications (cyclosporin A, methotrexate) or JAK-inhibitors [Citation10]. However, our patient didn’t wish immediate initiation of the new systemic therapy, but further reevaluation, if topical treatment would be unsuccessful. Thus, he has been treated with medium potent TCS and TCI and for both psoriasis and atopic dermatitis and reported currently a stable condition.
Statement on prior presentation
The contents of this manuscript have not been previously published and are not currently submitted elsewhere.
Disclosure statement
Galina Balakirski has been an advisor, speaker or investigator for Abbvie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen-Cilag, Leo Pharma, Novartis, Pfizer, Sanofi, UCB Pharma.
Sven-Niklas Burmann has no conflicts of interest to declare.
Silke C. Hofmann has been an advisor, speaker or investigator for Amgen, Janssen-Cilag, Novartis, Infectopharm, and GSK.
Alexander Kreuter has been an advisor, speaker or investigator for Sanofi, Almirall, Boehringer Ingelheim, Infectopharm, and MSD Sharp & Dohme.
Additional information
Funding
References
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:1–3. doi: 10.1016/j.coi.2017.08.008.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis - a retrospective review. J Dermatolog Treat. 2021;32(7):716–720. doi: 10.1080/09546634.2019.1702147.
- Al-Janabi A, Foulkes AC, Mason K, et al. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol. 2020;34(7):1440–1448. doi: 10.1111/jdv.16246.
- Brumfiel CM, Patel MH, Zirwas MJ. Development of psoriasis during treatment with dupilumab: a systematic review. J Am Acad Dermatol. 2022;86(3):708–709. doi: 10.1016/j.jaad.2021.05.013.
- Hahn M, Ghoreschi K. The role of IL-4 in psoriasis. Expert Rev Clin Immunol. 2017;13(3):171–173. doi: 10.1080/1744666X.2017.1279054.
- Quattrini L, Caldarola G, Falco GM, et al. Successful treatment with tralokinumab in patients with atopic dermatitis and dupilumab-induced psoriasis. J Eur Acad Dermatol Venereol. 2023. doi: 10.1111/jdv.19351.
- Mirza FN, Wang A, Ramachandran SM, et al. Dupilumab-induced phenotype switch from atopic dermatitis to psoriasis is characterized by de novo interleukin-17A expression: a case report. Br J Dermatol. 2021;185(2):432–434. doi: 10.1111/bjd.20064.
- Colonna C, Bortoluzzi P, Cavalli R. Dupilumab treatment for severe atopic dermatitis in children and SARS-CoV-2 infection: a combination of triggers for psoriasis. J Eur Acad Dermatol Venereol. 2023;37(5):e568–e569. doi: 10.1111/jdv.18864.
- Barry K, Zancanaro P, Casseres R, et al. A retrospective review of dupilumab and psoriasis biologic combination therapy. J Dermatolog Treat. 2021;32(4):438–439. doi: 10.1080/09546634.2019.1659481.
- Tsai YC, Tsai TF. Overlapping features of psoriasis and atopic dermatitis: from genetics to immunopathogenesis to phenotypes. Int J Mol Sci. 2022;23(10):5518. doi: 10.3390/ijms23105518.

