Abstract
Background
Available network meta-analyses (NMAs) comparing the efficacy of biologics in nail psoriasis (NP) have not included recently approved biologics such as bimekizumab nor have they provided comparisons up to 1 year.
Objective
We conducted two NMAs that update and extend results from a previous NMA comparing biologics for achieving complete resolution of NP.
Methods
Bayesian NMAs were performed using a generalized linear model with a logit link to model the binary outcome of nail clearance at weeks 24–28 and 48–52.
Results
For the NMA at weeks 24–28, which included seven biologics and placebo, the absolute probability of achieving complete resolution of NP was highest for ixekizumab (46.4%; 95% credibility interval [CrI] 35.2–58.0), followed by brodalumab (37.1%; 95% CrI 17.1–62.2) and bimekizumab (30.3%; 95% CrI 12.7–53.9). For the NMA at weeks 48–52, which included six biologics, the absolute probability was highest for ixekizumab (77.2%; 95% CrI 51.1–93.4), followed by adalimumab (75.6%; 95% CrI 61.5–87.3) and brodalumab (71.9%; 95% CrI 38.4–93.2).
Conclusion
Among biologics included in these two NMAs, ixekizumab has the highest absolute probability of achieving complete resolution of NP. Results may help to inform treatment decisions for patients with NP.
Introduction
Nail psoriasis (NP) affects up to half of patients with psoriasis and most patients with psoriatic arthritis (Citation1–4). NP is widely acknowledged as challenging to treat and, along with scalp psoriasis, psoriasis severity and other factors, it is an important predictor of the future development of psoriatic arthritis (Citation5, Citation6). Biologics have proven efficacious in the treatment of NP, although limited comparative information is available and few network meta-analyses (NMAs) that compared the efficacy of biologic treatments have been performed (Citation7–10). Moreover, available NMAs have not included recently approved biologics such as bimekizumab nor have they provided long-term comparisons up to 1 year. We conducted two NMAs that update and extend results from a previous NMA comparing biologics for achieving complete resolution of NP (Citation9), herein providing new comparative efficacy data that include bimekizumab and secukinumab and long-term outcomes up to 52 weeks.
Methods
Methods have been previously described by Reich et al. (Citation9). Briefly, the analysis included phase III randomized controlled trials of licensed biologics that focused on adults with moderate-to-severe chronic plaque psoriasis and concomitant fingernail psoriasis (Supplementary Table S1). Eligible clinical trials were identified from a targeted literature search conducted in May 2023 (with no specified start date for searches) and hand-searched from the bibliographies of published NMAs. The most recently reported clinical trial results included in the updated and extended analyses were from March 2023 (Citation11–24). Similar to previous analyses (Citation25), data were also extracted from posters (Citation21, Citation23) in the absence of full manuscripts. Outcomes of interest in the analyses were the proportion of patients that achieved complete resolution of NP at weeks 24–28 in the updated NMA and at weeks 48–52 in a new/second NMA independent of the shorter-term analysis. Complete resolution of NP was defined as a score of zero on the Nail Psoriasis Severity Index (NAPSI), modified NAPSI or Physician’s Global Assessment of Fingernails (PGA-F). Characteristics of the trials included in the updated NMA are provided in Supplementary Table S2.
Comparators
Treatments included the anti-tumor necrosis factor (TNF) agent adalimumab (initial dose of adalimumab 80 mg, followed by 40 mg every second week starting 1 week later), the interleukin (IL)-17A and F inhibitor bimekizumab (320 mg every 4 weeks [BE VIVID trial]; 320 mg every 4 weeks until week 16 then rerandomized to 320 mg every 4 weeks or every 8 weeks [BE RADIANT trial]), the IL-17 receptor A inhibitor brodalumab (210 mg, at weeks 0, 1, 2, and every 2 weeks thereafter), the IL-23p19 inhibitor guselkumab (100 mg, at weeks 0, 4, and every 8 weeks thereafter), the anti-TNF agent infliximab (5 mg/kg at weeks 0, 2, 6, and every 8 weeks thereafter), the IL-17A inhibitor ixekizumab (160 mg starting dose at week 0, followed by 80 mg every 2 weeks from week 2–12 and every 4 weeks thereafter), the IL-17A inhibitor secukinumab (300 mg every 1 week until week 4 then every 4 weeks), and the IL-12/23p40 antibody ustekinumab (45/90 mg weight-based dosing at weeks 0, 4, and every 12 weeks thereafter) (Supplementary Table S1).
Statistical analysis
The NMAs were conducted in the Bayesian framework and performed with R Statistical Software (v3.4.2; R Core Team 2021) using the R2jags package. A generalized linear NMA model with a logit link was used to model the binary outcome of nail clearance at weeks 24–28 and 48–52. Both fixed and random-effect models were assessed, and the best model was selected based on deviance information criterion (lower values indicating better fit) and comparing the number of data points to the residual deviance (larger differences between the two indicating poorer fit). Convergence for all models was assessed using trace plots as modified by Brooks and Gelman (Citation26). Results are presented as a point estimate (posterior probability of nail clearance) and associated 95% credible intervals (CrI). For each outcome, the probability of each treatment being ranked best is also presented (Citation27). To assess the potential impact of a zero-cell count event in Elewski et al. (Citation13) on the overall week 24–28 NMA, a sensitivity analysis was performed following the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Citation28). In this analysis, a fixed value of 1.0 was added to both the adalimumab and placebo arm before re-running the NMA to account for computational problems when no events are observed in one group of an individual study (Citation28).
Results
Nail resolution NMA at weeks 24–28
A total of eight clinical trials provided comparative outcomes data at weeks 24–28 that connected to form an evidence network (). These phase III trials included two placebo-controlled trials (Citation13, Citation14) and six head-to-head active comparator trials (Citation11, Citation16–18, Citation20, Citation21) evaluating a total of seven active biologic treatments plus placebo.
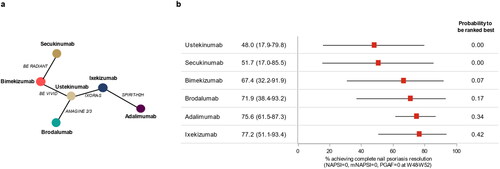
Figure 1. Network diagram (a) and forest plot of absolute treatment effects (with 95% credibility intervals) (b) for complete resolution of nail psoriasis at weeks 24–28.
mNAPSI, modified NAPSI; NAPSI, Nail Psoriasis Severity Index; PGA-F, Physician’s Global Assessment of Fingernail Psoriasis.
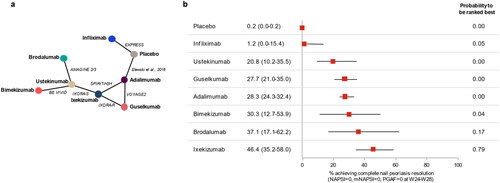
The absolute probability of achieving complete resolution of NP at weeks 24–28 was highest for ixekizumab at 46.4% (95% CrI 35.2–58.0), followed by brodalumab (37.1%; 95% CrI 17.1–62.2), bimekizumab (30.3%; 95% CrI 12.7–53.9), adalimumab (28.3%; 95% CrI 24.3–32.4), guselkumab (27.7%; 95% CrI 21.0–35.0), ustekinumab (20.8%; 95% CrI 10.2–35.5), and infliximab (1.2%; 95% CrI 0.0–15.4) (). Aside from infliximab, all biologics were significantly better at achieving nail clearance than placebo. The Bayesian NMA also showed that ixekizumab had the highest absolute probability to be ranked best (79%), followed by brodalumab (17%) and bimekizumab (4%). Findings of the sensitivity analysis using a continuity correction for zero-event studies were largely consistent with the principal analysis for the top-ranking biologics. However, the response rate for infliximab was 23.8% higher than in the main analysis, resulting in a reversal of the order with ustekinumab (Supplementary Figure S1).
Nail resolution NMA at weeks 48–52
The evidence network for outcomes data at weeks 48–52 included five clinical trials, all head-to-head comparative studies (Citation11, Citation17, Citation18, Citation21, Citation23), involving six different interventions (). For this network, the absolute probability of achieving complete resolution of NP at weeks 48–52 was highest for ixekizumab at 77.2% (95% CrI 51.1–93.4), followed by adalimumab (75.6%; 95% CrI 61.5–87.3), brodalumab (71.9%; 95% CrI 38.4–93.2), bimekizumab (67.4%; 95% CrI 32.2–91.9), secukinumab (51.7%; 95% CrI 17.0–85.5), and ustekinumab (48.0%; 95% CrI 17.9–79.8) (). Based on the Bayesian NMA, ixekizumab was associated with the highest absolute probability to be ranked best (42%), followed by adalimumab (34%), brodalumab (17%), bimekizumab (7%), secukinumab (0%), and ustekinumab (0%).
Discussion
These NMAs compared the efficacy of biologic treatments in achieving complete resolution of NP in patients with moderate-to-severe psoriasis and concomitant fingernail psoriasis. Results showed that ixekizumab had the highest probability of achieving complete resolution of NP at weeks 24–28 in the updated NMA and at weeks 48–52 in the new/extended analysis. For biologics included in both the short- and long-term NMAs, the probability of achieving complete resolution of NP was higher overall in the long-term NMA, although differences between biologic treatments were smaller (and not statistically significant) than in the short-term NMA, potentially indicating that duration of treatment regardless of the neutralized cytokine pathway is a key determinant of treatment success (Citation29).
Results of this study update and extend findings of the NMA conducted by Reich et al. (Citation9), which showed ixekizumab had the highest probability of achieving complete resolution of NP and of being ranked best at weeks 24–26, being consistent with other NMAs (Citation7, Citation8, Citation10). In the updated NMA at weeks 24–28, the ranked order from highest to lowest absolute probability of achieving complete resolution of NP was the same as in the original NMA for the biologics included. In addition to the clinical trial data used in the previous NMA, our updated analysis included clinical outcomes data for the recently approved biologic bimekizumab in the shorter-term nail resolution NMA at 24–28 weeks; bimekizumab was among the top three biologics evaluated at this period. The new/extended analysis also provides comparative efficacy data for six biologics in a long-term nail resolution NMA at 48–52 weeks. The evidence network for this NMA included data from two recently reported head-to-head comparative trials with bimekizumab (Citation21, Citation23) and secukinumab (Citation23). Unfortunately, we were unable to include other modern biologics, such as risankizumab (Citation30) and tildrakizumab (Citation31) in our NMAs, as the authors are not aware of any head-to-head or placebo-controlled studies that have reported on complete resolution of NP at the study time points required for integration into the network.
To the best of our knowledge this is the first published NMA providing short- and long-term (up to 1 year) relative comparisons of biologic efficacy in achieving complete resolution of NP at the same time. The study also includes two biologics not included in previous NMAs—bimekizumab in both the short- and long-term NMA, and secukinumab in the long-term NMA. In addition, the analysis focused on complete resolution of NP as the key outcome. Complete nail resolution is a common outcome to multiple trials and only minimally affected by the scale or scoring system used (Citation9, Citation20). It is also the least subjective and ambiguous outcome in NP and arguably the most important treatment goal for physicians and patients. We intend to continue to monitor NP disclosures/publications and update our NMAs on a regular basis as new information becomes available, in a similar way as other “living” literature reviews/NMA updates (Citation32).
The study has several limitations. Studies included in the updated and extended NMAs, like the previous NMA conducted by Reich et al. (Citation9), were identified using a targeted literature review rather than a systematic literature review (SLR). However, the authors have validated their search results against other NP NMAs that used SLRs (Citation7, Citation8) and did not identify any additional clinical trials from those NMAs that were not included in our NMAs. Severity of NP varied across studies in the NMA; therefore, some patients may have had relatively mild disease at baseline and their improvement may not have been clinically significant. Ixekizumab has demonstrated similar efficacy in achieving complete resolution of NP regardless of the level of NP severity at baseline in adults (Citation33) and pediatric patients (≥6 years old) (Citation34), although this effect has not been reported (i.e., is unknown) for most other biologics. In IXORA-S, the efficacy of ixekizumab on NP clearance was only minimally affected irrespective of whether there was significant NP (defined as NAPSI ≥16 and ≥4 fingernails involved) or not (Citation17). It can be assumed that studies with a higher degree of NP probably lead to an underestimation of the calculated response rate for drugs from these studies, although the exact magnitude of this effect cannot be ascertained and needs to be estimated in future studies. Other cross-trial differences in study design and patient characteristics may also influence the treatment effect and introduce bias in the comparisons. Missing data were also handled differently, potentially leading to an overestimation of treatment effects in studies using multiple imputation or as observed rather than non-responder imputation. It can be assumed that studies employing non-responder imputation tend to underestimate the true response rate, whereas studies reporting multiple imputation or as observed results tend to overestimate the treatment effect of the investigated biologic (a factor that becomes more pertinent in long-term analyses). Another limitation is that the new information included in the NMA for bimekizumab was derived from clinical trials that used more frequent administration than the approved/labelled regimen for bimekizumab (Citation35), potentially overestimating the treatment effect of this biologic. In addition, the relevant data from the bimekizumab clinical trials (BE VIVID and BE RADIANT) have been reported in posters (Citation21, Citation23) and are not fully published, although the use of data from posters is not unprecedented in NMAs (Citation25). Finally, the study was somewhat limited by the scarcity of clinical trial data and low sample sizes of patients also affected by NP, which in some cases resulted in wide 95% CrIs. In particular, the treatment effect of infliximab was likely underestimated in the shorter-term NMA because of the connection to the network via placebo through the EXPRESS trial (Citation14) and Elewski et al. (Citation13), with the latter having a placebo response rate of 0%. This relatively low placebo response in the trial with adalimumab (Citation13) compared with the EXPRESS trial with infliximab (Citation14) was likely because patients entering the adalimumab trial were required to have more severe NP, whereas those entering the infliximab trial could have any degree of NP severity. A sensitivity analysis was run to account for this zero-count event and the computational issues associated with it (Citation28). While this sensitivity analysis did not alter the ranking and performance of the top five biologics, confirming the robustness of the principal analysis, it resulted in the reversal of the order of ustekinumab and infliximab. As a result, the estimated response rate for infliximab aligns closely with what has been reported in the EXPRESS trial (Citation14).
In conclusion, this updated NMA showed that, among biologic treatments included in the analysis, ixekizumab has the highest probability of achieving complete resolution of NP. Results of this study may help to inform treatment decisions for patients with NP.
Supplemental Material
Download PDF (349.6 KB)Acknowledgments
The authors would like to acknowledge Greg Plosker (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript. Statistical analysis support for the study was also provided by Christophe Sapin, Michael Sonksen and Alan Brnabic (Eli Lilly and Company).
Disclosure statement
A. Egeberg has received research funding from Pfizer, Eli Lilly, Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, Boehringer Ingelheim, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from Amgen, AbbVie, Almirall, Leo Pharma, Zuellig Pharma Ltd., Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Union Therapeutics, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, McNeil Consumer Healthcare, Horizon Therapeutics, Boehringer Ingelheim, and Janssen Pharmaceuticals. Lars Erik Kristensen has received speaker & consultancy fees from AbbVie, Amgen, UCB, Galapagos, Novartis, Eli Lilly, Pfizer, Janssen Pharmaceutical, Zuellig Pharma. And IIT grants from AbbVie, UCB, Novartis, Eli Lilly, Pfizer. Luis Puig has received consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Biogen, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Fresenius-Kabi, Janssen, JS Biocad, Leo-Pharma, Lilly, Novartis, Pfizer, Samsung-Bioepis, and UCB. Phoebe Rich has received research grants as a principal investigator on pharmaceutical trials from AbbVie, Arcutis, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, Novartis, Sun Pharma, and UCB. Saxon D. Smith has served as an investigator, speaker, and/or advisor for AbbVie, Sanofi Aventis, Eli Lilly, Novartis, UCB, Regeneron, BMS, Pfizer, Sanofi Genzyme, Leo Pharma, Merck Sharp & Dohme Corp, Amgen, Janssen Cilag, Johnson and Johnson. Alyssa Garrelts, Kyoungah See, Thorsten Holzkaemper, Konstantinos Fotiou, Christopher Schuster are employees and minor stockholders of Eli Lilly and Company.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Rigopoulos D, Stathopoulou A, Gregoriou S. Small molecules and biologics in the treatment of nail psoriasis. Skin Appendage Disord. 2020;6(3):1–5. doi:10.1159/000507298.
- Sobolewski P, Walecka I, Dopytalska K. Nail involvement in psoriatic arthritis. Reumatologia. 2017;55(3):131–135. doi:10.5114/reum.2017.68912.
- Haneke E. Nail psoriasis: clinical features, pathogenesis, differential diagnoses, and management. Psoriasis (Auckl). 2017;7:51–63. doi:10.2147/PTT.S126281.
- Jiaravuthisan MM, Sasseville D, Vender RB, et al. Psoriasis of the nail: anatomy, pathology, clinical presentation, and a review of the literature on therapy. J Am Acad Dermatol. 2007;57(1):1–27. doi:10.1016/j.jaad.2005.07.073.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population-based study. Arthritis Rheum. 2009;61(2):233–239. Erratum in: arthritis Rheum. 2010;62(4):574. PMID: 19177544; PMCID: PMC3061343. doi:10.1002/art.24172.
- Yan D, Ahn R, Leslie S, et al. Clinical and genetic risk factors associated with psoriatic arthritis among patients with psoriasis. Dermatol Ther (Heidelb). 2018;8(4):593–604. doi:10.1007/s13555-018-0266-x.
- Szebényi J, Gede N, Hegyi P, et al. Efficacy of biologics targeting tumour necrosis factor-alpha, interleukin-17 -12/23, -23 and small molecules targeting JAK and PDE4 in the treatment of nail psoriasis: a network meta-analysis. Acta Derm Venereol. 2020;100(18):adv00318. doi:10.2340/00015555-3640.
- Huang IH, Wu PC, Yang TH, et al. Small molecule inhibitors and biologics in treating nail psoriasis: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;85(1):135–143. doi:10.1016/j.jaad.2021.01.024.
- Reich K, Conrad C, Kristensen LE, et al. Network meta-analysis comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis. J Dermatolog Treat. 2022;33(3):1652–1660. doi:10.1080/09546634.2021.1892024.
- Husein-ElAhmed H, Husein-ElAhmed S. Bayesian network meta-analysis of head-to-head trials for complete resolution of nail psoriasis. Clin Exp Dermatol. 2023;48(8):895–902. Apr 13:llad136. doi:10.1093/ced/llad136.
- Elewski B, Rich P, Lain E, et al. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: results from three phase 3 trials. J Dermatolog Treat. 2022;33(1):261–265. doi:10.1080/09546634.2020.1749546.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi:10.1056/NEJMoa1503824.
- Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J Am Acad Dermatol. 2018;78(1):90–99.e1. doi:10.1016/j.jaad.2017.08.029.
- Rich P, Griffiths CE, Reich K, et al. Baseline nail disease in patients with moderate to severe psoriasis and response to treatment with infliximab during 1 year. J Am Acad Dermatol. 2008;58(2):224–231. doi:10.1016/j.jaad.2007.07.042.
- Blauvelt A, Papp K, Gottlieb A, IXORA-R study group., et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358. doi:10.1111/bjd.18851.
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab versus guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomised, double-blinded trial. Br J Dermatol. 2021;184(6):1047–1058. doi:10.1111/bjd.19509.
- Wasel N, Thac ID, French LE, et al. Ixekizumab and ustekinumab efficacy in nail psoriasis in patients with moderateto-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol Ther (Heidelb). 2020;10(4):663–670. doi:10.1007/s13555-020-00383-x.
- Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131. doi:10.1136/annrheumdis-2019-215386.
- Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis. 2020;79(10):1310–1319. doi:10.1136/annrheumdis-2020-217372.
- Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018;154(6):676–683. doi:10.1001/jamadermatol.2018.0793.
- Papp KA, Lebwohl M, Gottlieb AB, et al. Bimekizumab for the treatment of moderate to severe plaque psoriasis with scalp, nail and palmoplantar involvement through 52 weeks: post-hoc analysis from the BE VIVID phase 3 trial. Poster presented at: european Academy of Dermatology and Venereology (EADV) Virtual Congress; 2020 Oct 29–31.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi:10.1016/S0140-6736(21)00125-2.Erratum in: lancet. 2021;397:670.
- Eyerich K, Gottlieb AB, Piaserico S, et al. Bimekizumab versus secukinumab for the treatment of nail psoriasis in patients with moderate to severe plaque psoriasis: results from the BE RADIANT phase 3b trial. Poster session 43878 presented at: American Academy of Dermatology (AAD) Conference; 2023 March 17–21; New Orleans, LA.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–152. doi:10.1056/NEJMoa2102383.
- Warren RB, See K, Burge R, et al. Rapid response of biologic treatments of moderate-to-severe plaque psoriasis: a comprehensive investigation using bayesian and frequentist network meta-analyses. Dermatol Ther (Heidelb). 2020;10(1):73–86. doi:10.1007/s13555-019-00337-y.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graphical Stat. 1998;7:434–455.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London (UK): National Institute for Health and Care Excellence (NICE); 2014. www.ncbi.nlm.nih.gov/books/NBK310366.
- Higgins JPT, Thomas J, Chandler J, et al. editors. Cochrane handbook for systematic reviews of interventions, version 6.3. Chichester (UK): John Wiley & Sons; 2022.
- Rusk AM, Fleischer AB.Jr. In psoriasis treatment, greater improvement in skin severity predicts greater improvement in nail severity. J Dermatolog Treat. 2021;32(8):894–897. doi:10.1080/09546634.2020.1720578.
- Kristensen LE, Soliman AM, Papp K, et al. Effects of risankizumab on nail psoriasis in patients with active psoriatic arthritis: results from KEEPsAKE 1. J Eur Acad Dermatol Venereol. 2022;36(5):e389–e392. doi:10.1111/jdv.17931.
- Galluzzo M, Talamonti M, Cioni A, et al. Efficacy of tildrakizumab for the treatment of difficult-to-treat areas: scalp, nail, palmoplantar and genital psoriasis. J Clin Med. 2022;11(9):2631. doi:10.3390/jcm11092631.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;5(5):CD011535. PMID: 35603936; PMCID: PMC9125768. doi:10.1002/14651858.CD011535.pub5.
- Egeberg A, Kristensen LE, Vender R, et al. Sustained resolution of nail psoriasis through 5 years with ixekizumab: a post-hoc analysis from UNCOVER-3. Acta Derm Venereol. 2022;102:adv00787. doi:10.2340/actadv.v102.2269.
- Seyger MMB, Reich A, El Baou C, et al. Efficacy of ixekizumab on nail psoriasis in paediatric patients with moderate-to-severe psoriasis: a post hoc analysis from IXORA-PEDS. J Eur Acad Dermatol Venereol. 2021;35(12):e911–e913. doi:10.1111/jdv.17564.
- European Medicines Agency: bimekizumab (Bimzelx®) summary of product characteristics. cited 2023 May 9]. Available from: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf.