Dear Editor,
Alopecia areata (AA) is a chronic immune-mediated disorder characterized by non-scarring hair loss that can involve any hair-bearing site (Citation1,Citation2). AA pathogenesis depends on dysregulation of the immune system, with interferon-gamma (IFN-gamma), Interleukin-15 (IL-15), and Janus kinase (JAK) playing pivotal roles in promoting inflammation and hair follicle damage (Citation3,Citation4). In clinical trials, JAK inhibition has been shown to prevent and reverse hair loss in alopecia areata (Citation5–7).
Baricitinib is an oral JAK-inhibitor that has been approved for the treatment of severe alopecia areata (AA) after being evaluated in two phase-III randomized clinical trials (BRAVE-AA1 and BRAVE-AA2) (Citation5). To date, very limited data are available on the use of baricitinib in a real-world setting. The aim of this study was to assess the effectiveness and safety of baricitinib for AA in adults with severe alopecia areata, defined as a Severity of Alopecia Tool (SALT) ≥50 (more than 50% scalp hair loss).
Patients received baricitinib 4 mg once daily, and they were evaluated at weeks 0, 16, 24, and 36. The effectiveness of baricitinib was assessed in terms of mean SALT reduction at week 36 compared with baseline and as percentage of patients with a SALT ≤ 20 at each visit. Patients were screened for viral hepatitis and latent tuberculosis before starting the treatment with baricitinib. After 4 weeks and then every 16 weeks, the patients underwent routine blood tests, including blood cell count, hepatic enzymes, and lipid profile.
The demographic characteristics of our population at baseline are summarized in .
Table 1. Characteristics of our population at baseline.
Fifty patients from four Dermatology Units in Milan, Italy, were included. At baseline, the mean SALT score was 84.38 with a standard deviation (SD) of 20.42, and 30 patients (60%) had a very severe AA (SALT 95–100). Thirty-four patients completed 16 weeks of treatment to date, and 26.5% of them achieved a SALT ≤ 20. At weeks 24 and 36, this percentage increased to 38.1% and 54.6%, out of 24 and 11 total patients, respectively (). The mean SALT score decreased to 70.10 (SD 29.56), 56.24 (34.39), 41.86 (33.24), and 32.27 (29.61) at weeks 8, 16, 24, and 36, respectively. At week 36, the mean percentage change from baseline in the SALT score was −61.6%. Our study found a higher SALT20 response compared with data from the BRAVE-AA1 and two studies, which reported a SALT ≤ 20 at week 36 in 38.8% and 35.9% of patients receiving baricitinib 4 mg (compared with 54.6% in our experience) (Citation5). Similarly, at weeks 16 and 24, our patients achieved comparable or slightly better responses in terms of SALT ≤ 20 compared with phase-III clinical trials. We also analyzed the pattern of clinical responses to baricitinib. Among all patients who achieved a SALT20 response, 15.8% of our patients were considered early responders (achieving SALT20 between 4 and 12 weeks of treatment), 68.4% were gradual responders (between 12 and 36 weeks), and 15.8% were late responders, after receiving baricitinib for more than 36 weeks (). The clinical improvement of a 64-year-old woman with severe AA during the course of the therapy with baricitinib is shown in . Baricitinib was well-tolerated throughout the study without any adverse events (AEs) leading to discontinuation or serious AEs. The most reported AEs were hypercholesterolemia (10 patients) and creatine phosphokinase elevation (3).
Figure 1. (a) Percentages of patients who achieved an absolute SALT score of 20 or less throughout the study period; (b) patterns of clinical response to baricitinib, including early-, gradual- and late-responders.
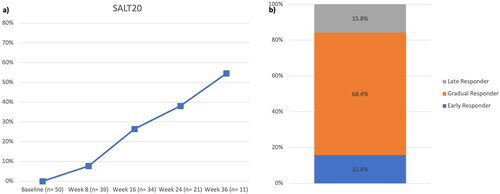
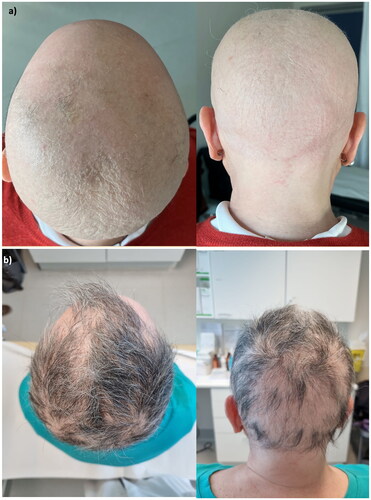
Figure 2. Clinical presentation of a 64-year-old woman before (a) starting baricitinib and after 36 weeks of treatment (b).
To our knowledge, this is one of the first real-world experiences evaluating the role of baricitinib for the treatment of severe AA. Despite a few limitations due to the retrospective nature of the study and the limited sample size, our results support data from phase-III clinical trials, with comparable patterns of clinical responses up to week 36. No new safety findings emerged from our study. The safety profile of baricitinib was comparable with other real-world experiences on JAK-inhibitors for atopic dermatitis (Citation8,Citation9). Longer experiences are required to further assess the effectiveness and safety of baricitinib in severe AA.
Ethical approval
Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice.
Consent form
All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
Disclosure statement
L. Gargiulo has been a consultant for Almirall. P. Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma and Almirall. S. M. Ferrucci has been principal investigator in clinical trials for ABBVIE, Almirall, Galderma, Leo Pharma, Sanofi, Amgen, Novartis, Bayer and received honoraria for lectures for Novartis and Menarini A. V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. M. Valenti has been a consultant and/or speaker for Sanofi, Leo Pharma, Eli Lilly, Novartis, Janssen, AbbVie and Boehringer Ingelheim. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme and UCB-Pharma. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. The other authors have nothing to declare.
Data availability statement
Additional data supporting the findings of this study are available from the Corresponding Author on reasonable request.
Additional information
Funding
References
- Pratt CH, King LEJr, Messenger AG, et al. Alopecia areata. Nat Rev Dis Primers. 2017;3(1):1. doi: 10.1038/nrdp.2017.11.
- Liu LY, King BA, Craiglow BG. Alopecia areata is associated with impaired health-related quality of life: a survey of affected adults and children and their families. J Am Acad Dermatol. 2018;79(3):556.e1–3. doi: 10.1016/j.jaad.2018.01.048.
- Meah N, Wall D, York K, et al. The alopecia areata consensus of experts (ACE) study: results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83(1):123–130. doi: 10.1016/j.jaad.2020.03.004.
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi: 10.1038/nm.3645.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–1699. doi: 10.1056/NEJMoa2110343.
- Kwon O, Senna MM, Sinclair R, et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2). Am J Clin Dermatol. 2023;24(3):443–451. doi: 10.1007/s40257-023-00764-w.
- Freitas E, Guttman-Yassky E, Torres T. Baricitinib for the treatment of alopecia areata. Drugs. 2023;83(9):761–770. doi: 10.1007/s40265-023-01873-w.
- Gargiulo L, Ibba L, Piscazzi F, et al. Effectiveness and safety of upadacitinib for moderate-to-severe atopic dermatitis in a real-world setting: a 52-week retrospective study. J Eur Acad Dermatol Venereol. 2023 [published online ahead of print, 2023 Sep 15]. doi: 10.1111/jdv.19507.
- Vittrup I, Elberling J, Skov L, et al. Short-term real-world experience with baricitinib treatment in danish adults with moderate-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37(4):e543–e546. doi: 10.1111/jdv.18804.

