Abstract
Background
This systematic review evaluated the Chinese herbal medicine (CHM) for treating atopic dermatitis (AD).
Methods
PubMed, EMBASE, the Cochrane library, the Wanfang database, and China National Knowledge Infrastructure (CNKI) were searched for relevant randomized controlled trials (RCTs) from inception to December 2021. Overall recovery rate, disease/symptom severity scoring, quality of life (QoL), recurrence rate, and incidence of adverse events (AEs) were evaluated. STATA SE 14.0 software was used for statistical analysis.
Results
17 RCTs involving 1624 patients were eligible. CHM was associated with a higher overall recovery rate (risk ratio [RR] = 1.15, 95% confidence interval [CI]: 1.05, 1.26, p = .003) and decreased recurrence rate (odds ratio [OR] = 0.19, 95% CI: 0.07, 0.55, p = .002), both confirmed by sensitivity analyses. CHM could decrease scoring atopic dermatitis index (MD = −0.61, 95% CI: −1.12, −0.11, p = .017), however, sensitivity analysis revealed non-robustness. No significant differences were found between the CHM and the control group in Eczema Area and Severity Index, QoL, and the incidence of AEs.
Conclusions
CHM was effective for treating AD as it could improve the overall recovery rate and decrease the recurrence rate. More studies are required to validate the potential of CHM on disease/symptoms severity and QoL.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by severe itchiness, skin redness and dryness, weeping, scarring, and lichenification (Citation1). Data from the previous study indicated that AD affects approximately 15% to 30% of children and 2% to 10% of adults (Citation2). More importantly, AD significantly affects daily living activities and leads to an increased financial burden for patients and their caregivers (Citation3). Therefore, establishing effective treatment for AD is of utmost importance.
At present, conventional treatments for AD continue to hold sway, such as topical therapies(topical corticosteroids, calcineurin inhibitors and antibiotics/antimicrobials), as well as systemic therapies(phototherapy, systemic corticosteroids, cyclosporine and methotrexate, etc) (Citation4). The former is mostly used for the first-line treatment of mild or severe AD patients while the latter is the main choice for severe and intractable ADs. In recent years, with the in-depth study of the pathological mechanism of AD, the use of small molecule targeted drugs and biologics has been widely concerned. JAK inhibitors (upadacitinib and abrocitinib), as representatives of small molecule targeted drugs, can improve the skin lesions and relieve itching in AD patients by blocking the common intracellular conduction pathways of various cytokines (Citation5). In addition, a large number of studies confirmed that the signal transduction mediated by interleukin (IL)-4 and IL-13 participated in the type 2 inflammatory reaction in the pathogenesis of AD, and IL-31, as a cytokine mainly produced by activated T cells, was also confirmed to be closely related to the pruritus of AD (Citation6). Based on the above researches, The United States Food and Drug Administration (FDA), the European Commission and the Japanese Dermatology Association (JDA) (Citation6) approved IL-4Rα inhibitor (Dupilumab), IL-13 inhibitor (tralokinumab) and IL-31Rα inhibitor (nemolizumab) respectively, in order to cope with AD patients with no obvious improvement after a period of common therapy, or the remaining intractable AD-related pruritus after a period of common therapy. Although the above therapies occupy the mainstream and are recognized by several national guidelines, the incidence of adverse events that cannot be ignored and the progress of drug resistance have always been a major concern for patients and health care professionals, such as glucocorticoid (Citation4). Therefore, it is of great significance to find drugs that are more effective, have fewer side effects or are suitable for a wider range of people.
As we all know, the application and treatment of traditional Chinese medicine in AD has been paid more and more attention internationally. The pathological formation of AD is multifactorial. Compared with modern medical preparations with a single component, oral or topical Chinese herbal medicine with multiple components may play a therapeutic role through multiple targets. For example, Hon found that the external washing formula of traditional Chinese medicine (honeysuckle, Polygonatum, licorice, etc.) had good antibacterial activity, especially for Staphylococcus aureus (Citation7). Ahn et al. showed that topical application of Jaungo ointment, which consists of Zicao (Radix arnebiae) and Danggui (Angelicae radix), could ameliorate the chronic-phase symptoms of AD (Citation8). Lee et al. reported that SSHT (a traditional Chinese medicine prescription, including Glycyrrhiza uralensis Fisch, Pinellia ternata, Bupleurum chinense DC, Bupleurum chinense DC, Ginseng DC, etc.) can relieve AD symptoms by regulating inflammatory mediators (Citation9). Two previously published systematic reviews on CHM for AD were inconclusive due to the poor quality of included studies and substantial statistical heterogeneity in selecting outcome measures (Citation10,Citation11). Therefore, it is necessary to comprehensively and systematically summarize and analyze the existing high-quality randomized controlled trials. This systematic review with meta-analysis aimed to further evaluate the therapeutic efficacy and safety of CHM for treating AD by comprehensively evaluating high-quality RCTs.
Material and methods
The present systematic review with meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) guideline (Citation12).
Search strategy
PubMed, EMBASE, the Cochrane library, the Wanfang database, and China National Knowledge Infrastructure (CNKI) were comprehensively searched for relevant studies from their inception until December 2021. We used the medical subject heading (MeSH) term of ‘Dermatitis, Atopic,’ ‘Medicine, Chinese Traditional,’ ‘Medicine, East Asian Traditional,’ ‘Herbal Medicine,’ ‘Drugs, Chinese Herbal,’ ‘Plants, Medicinal,’ and ‘Herbal Therapy’ as well as relevant keywords to develop the search strategy. The detailed search strategy of targeted English databases is summarized in Table S1.
Eligibility criteria
Inclusion criteria were as follows: (1) patients diagnosed with atopic dermatitis, or children/infants diagnosed with ‘eczema’; (2) intervention and control: Chinese herbal medicine + modern medicine vs. modern medicine alone; Chinese herbal medicine vs. placebo; (3) studies that reported at least one of the following outcomes: overall recovery rate (evaluated as cure and improvement rate), disease/symptom severity (evaluated by Eczema Area and Severity Index [EASI] or scoring atopic dermatitis index [SCORAD]); quality of life (QoL, evaluated by Dermatology Life Quality [DLQI]), recurrent rate, and the incidence of adverse events (AEs) at the last follow-up; 4) study design was limited to randomized controlled trial (RCT); 5) language was restricted to English or Chinese.
Exclusion criteria were the following: (1) repeated reports of the same study; (2) single-arm studies, case reports, case series, and reviews; and (3) studies with incomplete data.
Data extraction
Eligible studies were selected by two independent reviewers through two stages, which included screening titles and abstracts and checking full texts. Disagreements regarding study selection were resolved by consulting a third reviewer. The following data were extracted from each eligible study: author’s name, publication year, study design, country, sample size, mean age, diagnostic criteria, detailed interventions, dose, disease duration, follow-up duration, and outcomes.
Risk of bias assessment
We assessed the risk of bias in the included studies by using the seven items from the Cochrane risk of bias assessment tool (RoB) (Citation13), including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias resources. The overall quality of each study was rated as ‘low’, ‘unclear’, or ‘high’ risk according to the assessment results.
Statistical analysis
The risk ratio (RR) with a 95% confidence interval (CI) was used to express the pooled results of dichotomous variables, including overall recovery rate, recurrence rate, and incidence of AEs. We used mean difference (MD) with a 95% CI to express the pooled results for continuous variables, including EASI, SCORAD, and QoL. We used Cochrane Q and I2 statistics to evaluate statistical heterogeneity across studies. When p < .10 and I2≥50%, statistically significant heterogeneity is considered to exist between studies and a random effects model would be used for meta-analysis. In contrast, the statistical heterogeneity across studies is insignificant if p ≥ .1 and I2<50%, then the fixed-effects model would be adopted for meta-analysis. We conducted a sensitivity analysis by introducing the leave-one-out method to examine the robustness of the pooled results. We also performed subgroup analysis regarding disease/symptoms severity according to the different measurements. In addition, more than ten studies reported an overall recovery rate as an outcome, allowing publication bias to be assessed by Egger’s and Begg’s tests. In all cases, p < .05 were considered statistically significant. STATA SE 14.0 software (Stata Corp, College Station, Texas, USA) was used to perform all statistical analyses.
Results
Identification of eligible studies
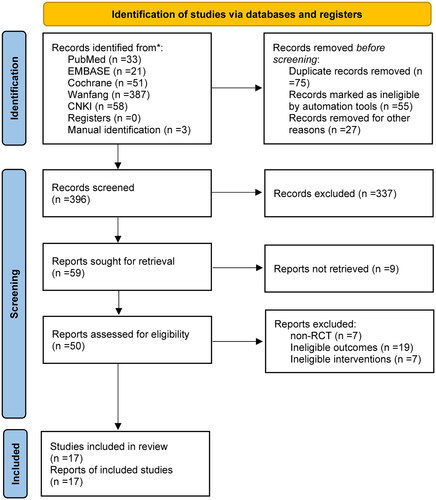
Initially, we identified 33 records from PubMed, 21 from EMBASE, 51 from the Cochrane library, 387 from Wanfang, and 58 from CNKI. Moreover, additional three studies were identified after checking the previous meta-analyses and the reference lists of included studies. After the initial removal of 157 duplicate records, 337 ineligible studies were excluded after screening titles and abstracts. After screening the full texts of 50 studies, 33 ineligible ones were excluded for the following reasons: non-RCTs (n = 7), lack of outcomes of interest (n = 14), and ineligible interventions (n = 7). Finally, 17 studies (Citation8,Citation9,Citation14–28) were included in this meta-analysis. The identification and selection of eligible studies are illustrated in .
Study characteristics
Among the 17 RCTs, 1624 patients with AD, atopic eczema (AE), or infantile eczema were finally included. The study characteristics are shown in . The mean age of the patients assigned to receive CHM ranged from 8.01 months to 38.6 years, compared to 7.65 months–39.6 years in those patients in the control group. The proportion of males varied from 16.7% to 68.6% in the CHM group and 11.2%–90.9% in the control group. The disease duration of patients in the CHM group ranged from 9 days to 17.5 years, and that in the control group ranged from 8 days to 18.2 years. Follow-up duration ranged from 2 to 24 weeks. Detailed baseline characteristics of each eligible study are summarized in .
Table 1. Baseline characteristics of 17 included studies.
Risk of bias assessment
According to the Cochrane risk of bias assessment criteria, five studies were rated as low risk, nine studies were rated as moderate risk, and the remaining three were rated as high risk. Detailed results of the risk of bias assessment of all studies are summarized in .
Table 2. Detailed results of risk of bias assessment.
Overall recovery rate
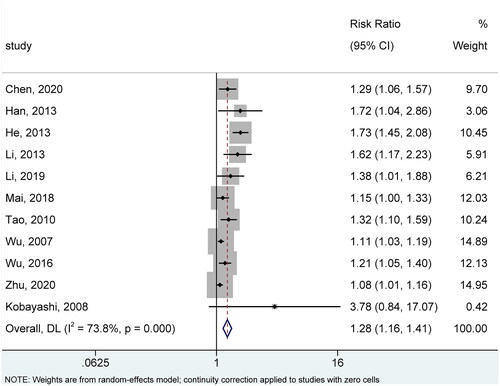
Eleven studies (Citation14,Citation16–20,Citation23–26,Citation28) reported overall recovery based on cure and improvement rate at the last follow-up. There was significant statistical heterogeneity across studies with reference to this outcome (I2=73.8%, p < .001). Therefore, we selected the random-effects model for meta-analysis, and the pooled result showed that CHMs were associated with a significantly improved overall recovery rate (RR = 1.28, 95%CI: 1.16, 1.41, p < .001) (). As shown in Figure S1, sensitivity analysis further substantiated the robustness of the pooled result.
Disease/symptoms severity
Six studies (Citation8,Citation9,Citation16,Citation20,Citation22,Citation28) used SCORAD to evaluate the disease/symptoms severity; however, there was significant statistical heterogeneity across studies in terms of this outcome (I2=87.1%, p < .001). Therefore, we selected the random-effects model for meta-analysis, and the pooled result suggested that CHMs significantly decreased the SCORAD compared to controls (MD= −0.61, 95% CI: −1.12, −0.11, p = .017) (). However, as shown in Figure S2, the pooled result was significantly changed after removing the study by Li et al. (Citation20).
Figure 3. Meta-analysis of disease/symptoms severity based on (A) SCORAD and (B) EASI. MD: mean difference; SCORAD: scoring atopic dermatitis index; EASI: Eczema Area and Severity Index.

Six studies (Citation8,Citation14,Citation15,Citation19,Citation21,Citation27) evaluated disease/symptoms severity using EASI; however, there was significant statistical heterogeneity across studies concerning this outcome (I2=87.2%, p < .001). Thus, we selected the random-effects model for meta-analysis, and the pooled result showed no significant difference between the two groups (MD = −0.55, 95% CI: −1.24, 0.13, p = .113) (). However, after removing the study by Chen et al. (Citation14), the pooled result was significantly changed to benefit CHMs, as shown in Figure S3.
Recurrence rate
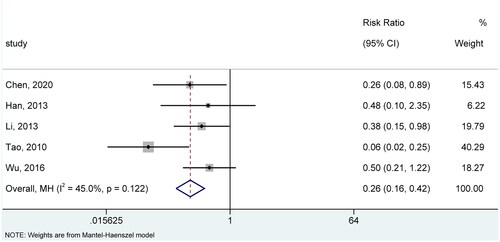
Among 17 included studies, 5 (Citation14,Citation16,Citation19,Citation24,Citation26) evaluated the recurrence rate after treatment. There was insignificant statistical heterogeneity across studies regarding this outcome (I2=45.0%, p = .122). Therefore, we selected the fixed-effects model for meta-analysis. The pooled result suggested that patients receiving CHMs experienced fewer recurrences compared to patients receiving controls (RR = 0.26, 95% CI: 0.16, 0.42, p < .001) (). Sensitivity analysis further substantiated the robustness of the pooled result as it did not significantly change after removing each study (Figure S4).
Incidence of AEs
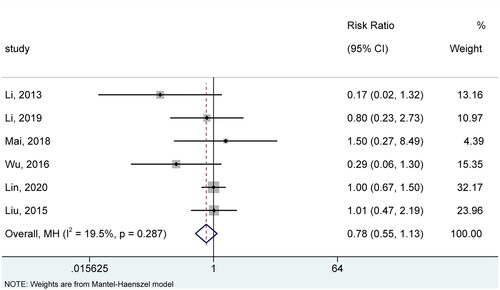
Six studies (Citation19–23,Citation26) reported the incidence of AEs. As there was insignificant statistical heterogeneity across studies regarding this outcome (I2=19.5%, p = .287), we selected the fixed-effects model for meta-analysis. The pooled result suggested no significant difference between the two groups in the incidence of AEs (RR =0.78, 95% CI: 0.55, 1.13, p = .187) (). Sensitivity analysis further substantiated the robustness of the pooled result as it did not significantly change after removing each study (Figure S5).
QoL
Among 17 included studies, 2 (Citation15,Citation21) evaluated the QoL using DLQI; however, there was no significant statistical heterogeneity across studies regarding this outcome (I2=0.0%, p = .702). Therefore, we selected the fixed-effects model for meta-analysis. The pooled result revealed no significant difference between the two groups (MD = −0.18, 95% CI: −0.64, 0.29, p = .459), and sensitivity analysis did not significantly change the pooled result (Figure S6).
Publication bias
After conducting Egger’s and Begg’s tests, we detected that the pooled result of the overall recovery rate (Egger’s test: t = 4.10, p = .003; Begg’s test: z = 2.49, p = .013) might be affected by the publication bias; however, publication bias was absent for SCORAD (Egger’s test: t = 2.25, p = .110; Begg’s test: z = 0.38, p = .707), EASI (Egger’s test: t = 1.77, p = .151; Begg’s test: z = 1.13, p = .260), recurrence rate (Egger’s test: t = −1.02, p = .383; Begg’s test: z = 0.73, p = .462), and the incidence of AEs (Egger’s test: t = −1.57, p = .192; Begg’s test: z = 1.50, p = .133). In addition, a publication bias examination was not conducted for QoL due to insufficient studies.
Discussion
This systematic review with meta-analysis revealed that CHM, in combination with modern medicine, could improve the overall recovery rate and decrease the recurrence rate among patients with AD, AE, and eczema. However, the therapeutic potential of CHM on disease/symptoms remained uncertain. In addition, CHM did not reduce the incidence of AEs and improve QoL compared to modern medicine or placebo.
The complementary or alternative treatment strategy of traditional Chinese medicine in treating atopic rash was mainly selected by Chinese doctors, so we referred to the Consensus of experts in TCM diagnosis and treatment of eczema (Citation29). The guide showed that the diagnosis and treatment of atopic dermatitis in traditional Chinese medicine can be roughly divided into two aspects, the internal treatment and the external treatment. The Internal treatment refers to the treatment of etiology (oral administration is mostly used), aiming at regulating physical fitness, improving overall recovery rate, reducing recurrence rate and improving quality of life. Drugs such as honeysuckle, Polygonatum sibiricum, Poria and Forsythia suspensa are frequently used in this respect and are representative drugs for internal treatments. AD is a chronic inflammatory skin disease, in which the immune balance between Th1 and Th2 is a part of the pathogenesis. Studies have found that Poria cocos protein is the main component of Poria cocos, which can activate Th1 immune response, inhibit Th2 cytokines and immunoglobulin E (Citation30), and inhibit the occurrence of AD-like dermatitis in NC/Nga mice treated with 2,4-dinitrofluorobenzene by regulating Th1 immune response. Hao et al. (Citation31) reported that forsythia suspensa extract significantly inhibited the proliferation of T lymphocytes and the synthesis of interleukin −4(Th2 cytokine) induced by glycine. Therefore, above traditional Chinese medicines may improve the skin lesions of AD by regulating the immune balance between Th1 and Th2. The external treatment is symptomatic treatment (the route of administration is mostly local external use), and the main purpose is to relieve symptoms. Common drugs are indigo naturalis, Lithospermum, Cortex Phellodendri, etc. Studies have shown that indigo naturalis can inhibit skin inflammation by inhibiting the production of cytokines, the activity of nuclear factor kappa B and the activation of mitogen-activated protein kinase (Citation32). Jaungo ointment (Radix Arnebiae and Radix Angelicae Sinensis, etc.) has been produced since 2013 as a medicine for treating dry skin, frostbite, mites, anal fissure and nasal dermatitis (Citation8). Recently, non-clinical studies have shown that Jaungo can play an anti-allergic and anti-inflammatory role in AD mouse model for two weeks (Citation32).Although the two treatments have their own emphases, they are not antagonistic, but often used jointly to play a role together.
Tan et al. evaluated the efficacy and safety of oral CHM for 432 AD patients based on seven RCTs published between 1992 and 2010 (Citation11). They performed qualitative analyses by comparing CHM combined with modern medicine (MM) versus MM alone and CHM versus placebo to assess disease or symptom severity scores, QoL, and reduction in concurrent treatments. Due to the poor quality of the included RCTs and statistical heterogeneity in outcome measurements, the data on overall clinical scoring, including SCORAD and EASI, pruritus score, QoL, and AEs, were not quantitatively summarized. Other efficacy indicators mentioned in our study, such as recurrence rate, were not described. They only conducted meta-analyses on erythema and surface damage scores, and the results favored CHM compared to placebo. In addition, they applied different herbal ingredients, CHM formulations, TCM theory, and delivery methods compared with our meta-analysis. Nevertheless, Tan et al. suggested that CHM significantly improves symptom severity of AD and is well tolerated, which was consistent with our findings. Moreover, our study included 17 RCTs with high quality that have been published recently, which might provide more updated and comprehensive CHM treatment information and reduce the bias in the analysis.
Gu et al. reviewed the topical application of CHM in AE patients (Citation10) in their meta-analysis. All included studies were published between 2003 and 2012 and conducted in China. A total of 34 CHM derived from plants, insects, and mineral substances under 12 CHM theory categories were covered. Among them, Huangbai (Cortex phellodendri) was the most used herb, followed by Huanglian (Rhizoma coptidis), She-chuangzi (Fructus cnidii), Zicao (Radix arnebiae), and Bingpian (Borneolum syntheticum). Moist dressing of CHM, oil-based CHM preparation, or ointment applications on the affected skin areas were reported. There were methodological weaknesses in the included ten RCTs. All RCTs were assessed as having a high risk of performance bias regarding the blinding of personnel and participants. Total effectiveness rate, i.e., a sum of recovery and significant improvement rate, after treatment with CHM versus conventional medications were compared. Their results indicated that the overall effects favored CHM, which was consistent with our conclusions. However, Gu et al. noted a high heterogeneity for this outcome assessment, and subgroup analysis revealed that corticosteroid creams used as control interventions might be one source of heterogeneity. They also showed no difference between the CHM and control groups in the outcome of the overall severity score. SCORAD and EASI scoring were only analyzed by two studies, which made quantitative data analysis impossible. Moreover, these studies showed fewer AEs in the CHM groups compared to the control groups, which was also contrary to our findings. Their study did not address itching severity, skin sebum, recurrence, or quality of life in AE patients.
The present study has some limitations. First, the sample size of this study was relatively small, which inevitably bias the reliability of results. However, meta-analysis can accumulate sample sizes to achieve higher statistical power than individual studies, thereby increasing the reliability of the pooled results. Second, CHM formulation, theory, and delivery methods greatly differed among the 17 RCTs, therefore inevitably introduce heterogeneity to affect the robustness of pooled results. Third, the patients were highly heterogeneous, as children and adults with AD, AE, and eczema participated in the study. More importantly, sensitivity analysis also showed significant changes in pooled results. Therefore, our findings should also be further validated in future studies. Fourth, treatment outcomes were evaluated by a variety of criteria, and there was a lack of reliable diagnostic criteria among the included studies, especially for patients of a wide range of ages. Furthermore, all eligible studies reported limited data on objective indicators, making a data synthesis impossible. Therefore, we suggest that future studies design objective indicators to further evaluate the effect of CHM on AD.
Conclusions
CHM combined with modern medicine for AD showed better effectiveness than modern medicine alone or placebo in the overall recovery rate and recurrence rate. Our findings may contribute to the management of dermatitis and eczema diseases. However, more high-quality studies are required to substantiate the therapeutic role of CHM in improving disease/symptoms severity as sensitivity analysis significantly changed the pooled results. Moreover, more high-quality RCTs with a large sample size are warranted to investigate the impact of CHM on QoL.
Ethical approval
This article is a meta-analysis. The data come from published articles and does not require ethical approval.
Author contributions
(I) Conception and design: Jinjing Jia; (II) Administrative support: Dacan Chen; (III) Provision of study materials or patients: Junfeng Liu, Xiumei Mo; (IV) Collection and assembly of data: Jinjing Jia; (V) Data analysis and interpretation: Jinjing Jia, Sherman X. Gu; (VI) Manuscript writing: (all authors); (VII) Final approval of manuscript: (all authors).
Supplemental Material
Download Zip (854.5 KB)Acknowledgment
We thank the Young Talent Support Project of Guangzhou Association for Science and Technology, and Young Elite Scientists Sponsorship Program by CAST (YESS20220609) for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Funding
References
- Chang C, Keen CL, Gershwin ME. Treatment of eczema. Clin Rev Allergy Immunol. 2007;33(3):1–10. doi: 10.1007/s12016-007-0033-8.
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4(37):1–191. doi: 10.3310/hta4370.
- Calabrese G, Licata G, Gambardella A, et al. Topical and conventional systemic treatments in atopic dermatitis: have they gone out of fashion? Dermatol Pract Concept. 2022;12(1):e2022155. doi: 10.5826/dpc.1201a155.
- Licata G, Tancredi V, Pezzolo E, et al. Efficacy and safeness of tralokinumab in patients with atopic dermatitis who developed conjunctivitis under dupilumab: a case series. J Eur Acad Dermatol Venereol. 2023;37(8). doi: 10.1111/jdv.19108.
- Saeki H, Akiyama M, Abe M, et al. English version of Japanese guidance for biologics in treating atopic dermatitis. J Dermatol. 2023;50(10):e311–e322. doi: 10.1111/1346-8138.16932.
- Hon KL, Leung TF, Ng PC, et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2007;157(2):357–363. doi: 10.1111/j.1365-2133.2007.07941.x.
- Ahn JH, Yun Y, Kim MH, et al. Exploring the efficacy and safety of topical Jaungo application in patients with atopic dermatitis: a pilot randomized, double-blind, placebo-controlled study. Complement Ther Med. 2018;40:22–28. doi: 10.1016/j.ctim.2018.07.007.
- Lee JH, Jo EH, Jung JY, et al. Efficacy and safety of Soshiho-tang in atopic dermatitis patients with gastrointestinal disorders: a double-blinded, randomized, and placebo-controlled clinical trial. J Ethnopharmacol. 2021;274:114006. doi: 10.1016/j.jep.2021.114006.
- Gu S, Yang AW, Li CG, et al. Topical application of Chinese herbal medicine for atopic eczema: a systematic review with a meta-analysis. Dermatology. 2014;228(4):294–302. doi: 10.1159/000360526.
- Tan HY, Zhang AL, Chen D, et al. Chinese herbal medicine for atopic dermatitis: a systematic review. J Am Acad Dermatol. 2013;69(2):295–304. doi: 10.1016/j.jaad.2013.01.019.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Chen SY, Shan JJ, Xu TH, et al. Clinical observation on 55 cases of chronic eczema in children with blood-deficiency syndrome treated by Chinese materia medica fumigant combined with desonide cream. J Tradit Chin Med Pediatr. 2020;16(3):55–58.
- Gu SX, Mo X, Zhang AL, et al. A Chinese herbal medicine preparation (Pei Tu Qing Xin) for children with moderate-to-severe atopic eczema: a pilot randomized controlled trial. Br J Dermatol. 2018;179(6):1404–1405. doi: 10.1111/bjd.16988.
- Han HJ, Guo F, Liu HX. Efficacy and safety observation of Chinese medicine bath joint tacrolimus ointment for the treatment of children with atopic dermatitis. Chinese journal of dermatology and venereology of integrated Traditional Chinese and Western medicine. 2013;12(3):164–166.
- He CL. Treatment of exudative eczema in 84 infants by external use of Wuhuang liquid. Treatment of exudative eczema in 84 infants by external use of Wuhuang liquid. Medical theory and practice. 2013;26(23):3170.
- Kobayashi H, Ishii M, Takeuchi S, et al. Efficacy and safety of a traditional herbal medicine, Hochu-ekki-to in the long-term management of Kikyo (delicate constitution) patients with atopic dermatitis: a 6-month, multicenter, double-blind, randomized, placebo-controlled study. Evid Based Complement Alternat Med. 2010;7(3):367–373. doi: 10.1093/ecam/nen003.
- Li J, Huang ZX, Li QH. Clinical observation of infantile eczema treated by external washing of Chinese medicine combined with desonide cream and vitamin E allantoin cream. New Clinical medicine in China. 2013;6(2):122–124.
- Li PY, Zhang GR, Liu HX. Effect of Chinese medicine bath combined with tacrolimus ointment on treating children with atopic dermatitis. Big Doctor. 2019;4(6):89–90, 126.
- Lin YK, Chang SH, Yang CY, et al. Efficacy and safety of indigo naturalis ointment in treating atopic dermatitis: a randomized clinical trial. J Ethnopharmacol. 2020;250:112477. doi: 10.1016/j.jep.2019.112477.
- Liu J, Mo X, Wu D, et al. Efficacy of a Chinese herbal medicine for the treatment of atopic dermatitis: a randomised controlled study. Complement Ther Med. 2015;23(5):644–651. doi: 10.1016/j.ctim.2015.07.006.
- Mai XW. Clinical analysis on the treatment of infantile eczema with traditional Chinese medicine and external use of homemade traditional Chinese medicine. Electron J Clin Med Lit. 2018;5(39):164,9.
- Tao P. Curative effect of Chinese medicine wet compress combined with hydrocortisone butyrate cream on infantile eczema. J Qiannan Ethnic Med Coll. 2010;23(02):85–86.
- Wu DS, Lian Y, Li Y, et al. A comparative study of adjuvant treatment for atopic dermatitis in infants with natural indigo. Primary Medical Forum. 2007;11(19):869–870.
- Wu SX. Clinical observation of external use of Chinese medicine in treating infantile eczema. Shenzhen J Integr Chin West Med. 2016;26(08):42–43.
- Yen CY, Hsieh CL. Therapeutic effect of Tzu-Yun ointment on patients with atopic dermatitis: a preliminary, randomized, controlled, Open-Label study. J Altern Complement Med. 2016;22(3):237–243. doi: 10.1089/acm.2015.0324.
- Zhu J, Chen J. Clinical study on Chinese herbal fumigation and washing combined with mustela stelatopia emollient cream for atopic dermatitis in children. New traditional Chinese medicine. 2020;52(6):85–87.
- D Q, L Y. Eczema (wet sores) TCM diagnosis and treatment expert consensus. Chinese Journal of Dermatovenereology of Integrated Traditional and Western Medicine. 2021;20(05):517–521.
- Lu YT, Kuan YC, Chang HH, et al. Molecular cloning of a Poria cocos protein that activates Th1 immune response and allays Th2 cytokine and IgE production in a murine atopic dermatitis model. J Agric Food Chem. 2014;62(13):2861–2871. doi: 10.1021/jf405507e.
- Hao Y, Li D, Piao X, et al. Forsythia suspensa extract alleviates hypersensitivity induced by soybean beta-conglycinin in weaned piglets. J Ethnopharmacol. 2010;128(2):412–418. doi: 10.1016/j.jep.2010.01.035.
- Kim IS, Kim DH, Yun CY, et al. A (S)-(+)-decursin derivative, (S)-(+)-3-(3,4-dihydroxy-phenyl)-acrylic acid 2,2-dimethyl-8-oxo-3,4-dihydro-2H,8H-pyrano[3,2-g]-chromen-3-yl-ester, attenuates the development of atopic dermatitis-like lesions in NC/Nga mice. Mol Biol Rep. 2013;40:2541–2548.